Going native: voltage-gated potassium channels controlling neuronal excitability
- PMID: 20519310
- PMCID: PMC2976014
- DOI: 10.1113/jphysiol.2010.191973
Going native: voltage-gated potassium channels controlling neuronal excitability
Abstract
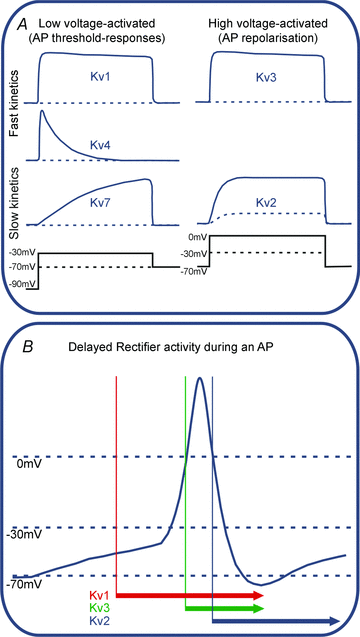
In this review we take a physiological perspective on the role of voltage-gated potassium channels in an identified neuron in the auditory brainstem. The large number of KCN genes for potassium channel subunits and the heterogeneity of the subunit combination into K(+) channels make identification of native conductances especially difficult. We provide a general pharmacological and biophysical profile to help identify the common voltage-gated K(+) channel families in a neuron. Then we consider the physiological role of each of these conductances from the perspective of the principal neuron in the medial nucleus of the trapezoid body (MNTB). The MNTB is an inverting relay, converting excitation generated by sound from one cochlea into inhibition of brainstem nuclei on the opposite side of the brain; this information is crucial for binaural comparisons and sound localization. The important features of MNTB action potential (AP) firing are inferred from its inhibitory projections to four key target nuclei involved in sound localization (which is the foundation of auditory scene analysis in higher brain centres). These are: the medial superior olive (MSO), the lateral superior olive (LSO), the superior paraolivary nucleus (SPN) and the nuclei of the lateral lemniscus (NLL). The Kv families represented in the MNTB each have a distinct role: Kv1 raises AP firing threshold; Kv2 influences AP repolarization and hyperpolarizes the inter-AP membrane potential during high frequency firing; and Kv3 accelerates AP repolarization. These actions are considered in terms of fidelity of transmission, AP duration, firing rates and temporal jitter. An emerging theme is activity-dependent phosphorylation of Kv channel activity and suggests that intracellular signalling has a dynamic role in refining neuronal excitability and homeostasis.
Figures



References
-
- Aldrich RW. Fifty years of inactivation. Nature. 2001;411:643–644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

