Sprouting capacity of lumbar motoneurons in normal and hemisected spinal cords of the rat
- PMID: 20519315
- PMCID: PMC2956897
- DOI: 10.1113/jphysiol.2010.190389
Sprouting capacity of lumbar motoneurons in normal and hemisected spinal cords of the rat
Abstract
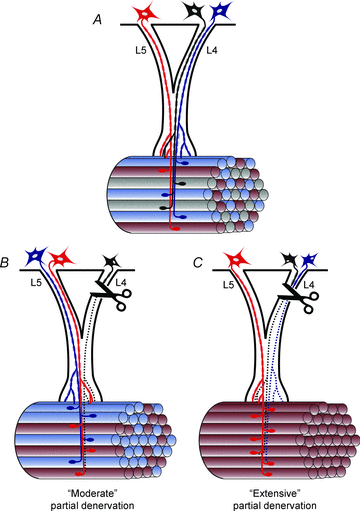
Nerve sprouting to reinnervate partially denervated muscles is important in several disease and injury states. To examine the effectiveness of sprouting of active and inactive motor units (MUs) and the basis for a limit to sprouting, one of three rat lumbar spinal roots was cut under normal conditions and when the spinal cord was hemisected at T12. Muscle and MU isometric contractile forces were recorded and muscle fibres in glycogen-depleted single muscle units enumerated 23 to 380 days after surgery. Enlargement of intact MUs by sprouting was effective in compensating for up to 80% loss of innervation. For injuries that removed >70-80% of the intact MUs, muscle contractile force and weight dropped sharply. For partial denervation of <70%, all MUs increased contractile force by the same factor in both normally active muscles and muscles whose activity was reduced by T12 hemisection. Direct measurements of MU size by counting glycogen-depleted muscle fibres in physiologically and histochemically defined muscle units, provided direct evidence for a limit in MU size, whether or not the activity of the muscles was reduced by spinal cord hemisection. Analysis of spatial distribution of muscle fibres within the outer boundaries of the muscle unit demonstrated a progressive increase in fibres within the territory to the limit of sprouting when most of the muscle unit fibres were adjacent to each other. We conclude that the upper limit of MU enlargement may be explained by the reinnervation of denervated muscle fibres by axon sprouts within the spatial territory of the muscle unit, formerly distributed in a mosaic pattern.
Figures



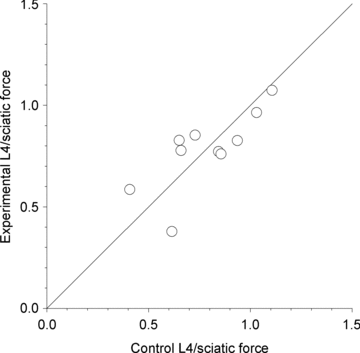


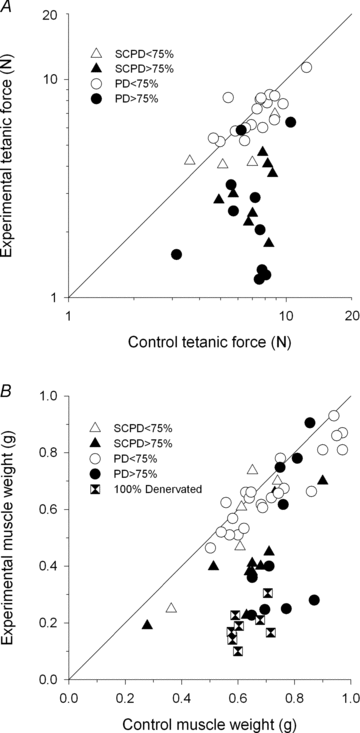

 spinal cords. The points that fall on the line of slope unity are those in which the partially denervated muscles develop as much force and are as heavy as those in the intact muscles. The points in Fig. 5 were averaged and the ±
spinal cords. The points that fall on the line of slope unity are those in which the partially denervated muscles develop as much force and are as heavy as those in the intact muscles. The points in Fig. 5 were averaged and the ±




References
-
- Bodine-Fowler SC, Garfinkel A, Roy RR, Edgerton VR. Spatial distribution of muscle fibres within the territory of a motor unit. Muscle Nerve. 1990;13:1133–1145. - PubMed
-
- Brooke MH, Kaiser KK. Muscle fibre types: how many and what kind. Arch Neurol. 1970;23:369–379. - PubMed
-
- Celichowski J, Krysciak K, Krutki P, Majczynski H, Gorska T, Slawinska U. Time-related changes of motor unit properties in the rat medial gastrocnemius muscle after the spinal cord injury. II. Effects of a spinal cord hemisection. J Electromyogr Kinesiol. 2009;20:532–541. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

