Melatonin potentiates glycine currents through a PLC/PKC signalling pathway in rat retinal ganglion cells
- PMID: 20519319
- PMCID: PMC2916991
- DOI: 10.1113/jphysiol.2010.187641
Melatonin potentiates glycine currents through a PLC/PKC signalling pathway in rat retinal ganglion cells
Abstract
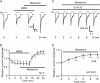
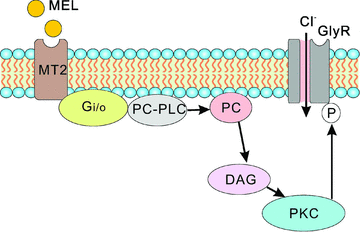
In vertebrate retina, melatonin regulates various physiological functions. In this work we investigated the mechanisms underlying melatonin-induced potentiation of glycine currents in rat retinal ganglion cells (RGCs). Immunofluorescence double labelling showed that rat RGCs were solely immunoreactive to melatonin MT(2) receptors. Melatonin potentiated glycine currents of RGCs, which was reversed by the MT(2) receptor antagonist 4-P-PDOT. The melatonin effect was blocked by intracellular dialysis of GDP-beta-S. Either preincubation with pertussis toxin or application of the phosphatidylcholine (PC)-specific phospholipase C (PLC) inhibitor D609, but not the phosphatidylinositol (PI)-PLC inhibitor U73122, blocked the melatonin effect. The protein kinase C (PKC) activator PMA potentiated the glycine currents and in the presence of PMA melatonin failed to cause further potentiation of the currents, whereas application of the PKC inhibitor bisindolylmaleimide IV abolished the melatonin-induced potentiation. The melatonin effect persisted when [Ca(2+)](i) was chelated by BAPTA, and melatonin induced no increase in [Ca(2+)](i). Neither cAMP-PKA nor cGMP-PKG signalling pathways seemed to be involved because 8-Br-cAMP or 8-Br-cGMP failed to cause potentiation of the glycine currents and both the PKA inhibitor H-89 and the PKG inhibitor KT5823 did not block the melatonin-induced potentiation. In consequence, a distinct PC-PLC/PKC signalling pathway, following the activation of G(i/o)-coupled MT(2) receptors, is most likely responsible for the melatonin-induced potentiation of glycine currents of rat RGCs. Furthermore, in rat retinal slices melatonin potentiated light-evoked glycine receptor-mediated inhibitory postsynaptic currents in RGCs. These results suggest that melatonin, being at higher levels at night, may help animals to detect positive or negative contrast in night vision by modulating inhibitory signals largely mediated by glycinergic amacrine cells in the inner retina.
Figures





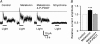
Similar articles
-
Orexin-A differentially modulates AMPA-preferring responses of ganglion cells and amacrine cells in rat retina.Neuropharmacology. 2015 Jun;93:80-93. doi: 10.1016/j.neuropharm.2015.01.016. Epub 2015 Feb 3. Neuropharmacology. 2015. PMID: 25656479
-
Orexin-A potentiates L-type calcium/barium currents in rat retinal ganglion cells.Neuroscience. 2015 Oct 1;305:225-37. doi: 10.1016/j.neuroscience.2015.08.008. Epub 2015 Aug 7. Neuroscience. 2015. PMID: 26259903
-
Melatonin inhibits tetraethylammonium-sensitive potassium channels of rod ON type bipolar cells via MT2 receptors in rat retina.Neuroscience. 2011 Jan 26;173:19-29. doi: 10.1016/j.neuroscience.2010.11.028. Epub 2010 Nov 18. Neuroscience. 2011. PMID: 21094224
-
Phosphatidylcholine-specific phospholipase C regulates thapsigargin-induced calcium influx in human lymphocytes.J Biol Chem. 1997 Dec 26;272(52):32861-8. doi: 10.1074/jbc.272.52.32861. J Biol Chem. 1997. PMID: 9407064
-
Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: a mini-review of literature.Neurochem Res. 2012 Apr;37(4):671-9. doi: 10.1007/s11064-011-0659-z. Epub 2011 Nov 22. Neurochem Res. 2012. PMID: 22101393 Free PMC article. Review.
Cited by
-
Group I mGluR-mediated inhibition of Kir channels contributes to retinal Müller cell gliosis in a rat chronic ocular hypertension model.J Neurosci. 2012 Sep 12;32(37):12744-55. doi: 10.1523/JNEUROSCI.1291-12.2012. J Neurosci. 2012. PMID: 22972998 Free PMC article.
-
GluA2 trafficking is involved in apoptosis of retinal ganglion cells induced by activation of EphB/EphrinB reverse signaling in a rat chronic ocular hypertension model.J Neurosci. 2015 Apr 1;35(13):5409-21. doi: 10.1523/JNEUROSCI.4376-14.2015. J Neurosci. 2015. PMID: 25834064 Free PMC article.
-
Melatonin modulates M4-type ganglion-cell photoreceptors.Neuroscience. 2015 Sep 10;303:178-88. doi: 10.1016/j.neuroscience.2015.06.046. Epub 2015 Jul 2. Neuroscience. 2015. PMID: 26141846 Free PMC article.
-
L- and T-type Ca2+ channels dichotomously contribute to retinal ganglion cell injury in experimental glaucoma.Neural Regen Res. 2023 Jul;18(7):1570-1577. doi: 10.4103/1673-5374.360277. Neural Regen Res. 2023. PMID: 36571364 Free PMC article.
-
Intravitreal Delivery of Melatonin Is Protective Against the Photoreceptor Loss in Mice: A Potential Therapeutic Strategy for Degenerative Retinopathy.Front Pharmacol. 2020 Feb 12;10:1633. doi: 10.3389/fphar.2019.01633. eCollection 2019. Front Pharmacol. 2020. PMID: 32116667 Free PMC article.
References
-
- Alarma-Estrany P, Pintor J. Melatonin receptors in the eye: location, second messengers and role in ocular physiology. Pharmacol Ther. 2007;113:507–522. - PubMed
-
- Barrenetxe J, Delagrange P, Martinez JA. Physiological and metabolic functions of melatonin. J Physiol Biochem. 2004;60:61–72. - PubMed
-
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994;40:3–29. - PubMed
-
- Cahill GM, Besharse JC. Light-sensitive melatonin synthesis by Xenopus photoreceptors after destruction of the inner retina. Vis Neurosci. 1992;8:487–490. - PubMed
-
- Cecchetti S, Spadaro F, Lugini L, Podo F, Ramoni C. Functional role of phosphatidycholine-specific phospholipases C in regulating CD16 membrane expression in natural killer cells. Eur J Immunol. 2007;37:2912–2922. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

