Guidance molecules in axon regeneration
- PMID: 20519341
- PMCID: PMC2890195
- DOI: 10.1101/cshperspect.a001867
Guidance molecules in axon regeneration
Abstract
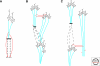
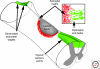
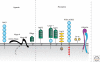
The regenerative capacity of injured adult mammalian central nervous system (CNS) tissue is very limited. Disease or injury that causes destruction or damage to neuronal networks typically results in permanent neurological deficits. Injury to the spinal cord, for example, interrupts vital ascending and descending fiber tracts of spinally projecting neurons. Because neuronal structures located proximal or distal to the injury site remain largely intact, a major goal of spinal cord injury research is to develop strategies to reestablish innervation lost as a consequence of injury. The growth inhibitory nature of injured adult CNS tissue is a major barrier to regenerative axonal growth and sprouting. An increasing complexity of molecular players is being recognized. CNS inhibitors fall into three general classes: members of canonical axon guidance molecules (e.g., semaphorins, ephrins, netrins), prototypic myelin inhibitors (Nogo, MAG, and OMgp) and chondroitin sulfate proteoglycans (lecticans, NG2). On the other end of the spectrum are molecules that promote neuronal growth and sprouting. These include growth promoting extracellular matrix molecules, cell adhesion molecules, and neurotrophic factors. In addition to environmental (extrinsic) growth regulatory cues, cell intrinsic regulatory mechanisms exist that greatly influence injury-induced neuronal growth. Various degrees of growth and sprouting of injured CNS neurons have been achieved by lowering extrinsic inhibitory cues, increasing extrinsic growth promoting cues, or by activation of cell intrinsic growth programs. More recently, combination therapies that activate growth promoting programs and at the same time attenuate growth inhibitory pathways have met with some success. In experimental animal models of spinal cord injury (SCI), mono and combination therapies have been shown to promote neuronal growth and sprouting. Anatomical growth often correlates with improved behavioral outcomes. Challenges ahead include testing whether some of the most promising treatment strategies in animal models are also beneficial for human patients suffering from SCI.
Figures

References
-
- Aguayo AJ, David S, Bray GM 1981. Influences of the glial environment on the elongation of axons after injury: Transplantation studies in adult rodents. J Exp Biol 95: 231–240 - PubMed
-
- Aguayo AJ, Dickson R, Trecarten J, Attiwell M, Bray GM, Richardson P 1978. Ensheathment and myelination of regenerating PNS fibres by transplanted optic nerve glia. Neurosci Lett 9: 97–104 - PubMed
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M 2008. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 322: 967–970 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
