Altered differentiation and proliferation of prostate epithelium in mice lacking the androgen receptor cofactor p44/WDR77
- PMID: 20519372
- PMCID: PMC2940529
- DOI: 10.1210/en.2009-1080
Altered differentiation and proliferation of prostate epithelium in mice lacking the androgen receptor cofactor p44/WDR77
Abstract
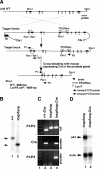
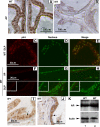
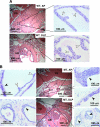
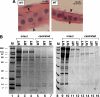
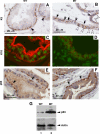
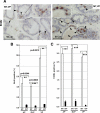
Although it has been observed that various cofactors modulate activity of the androgen receptor (AR), the specific relationship between AR cofactors and prostate development and functions has not been well studied. To determine whether AR cofactor p44/WDR77 is important in prostate growth and development, we examined prostate architecture in p44/WDR77-null mice and wild-type (WT) littermates. Prostate glands from p44/WDR77-deficient animals were not only smaller than those from WT mice but also had fewer branches and terminal duct tips and were deficient in production of secretory proteins. The p44/WDR77-null prostate tissue was less differentiated and hyperproliferative relative to WT littermates. In addition, the altered expression of androgen-regulated genes was observed in the p44/WDR77-null prostate. Thus, these results suggest that the AR cofactor p44/WDR77 plays important roles in prostate growth and differentiation by modulating AR-target gene expression.
Figures


Similar articles
-
Nuclear transport signals control cellular localization and function of androgen receptor cofactor p44/WDR77.PLoS One. 2011;6(7):e22395. doi: 10.1371/journal.pone.0022395. Epub 2011 Jul 15. PLoS One. 2011. PMID: 21789256 Free PMC article.
-
cGMP-dependent protein kinase Iβ interacts with p44/WDR77 to regulate androgen receptor-driven gene expression.PLoS One. 2013 Jun 3;8(6):e63119. doi: 10.1371/journal.pone.0063119. Print 2014. PLoS One. 2013. PMID: 23755100 Free PMC article.
-
Roles of the androgen receptor cofactor p44 in the growth of prostate epithelial cells.J Mol Endocrinol. 2006 Oct;37(2):283-300. doi: 10.1677/jme.1.02062. J Mol Endocrinol. 2006. PMID: 17032745
-
Androgen receptor coactivators that inhibit prostate cancer growth.Am J Clin Exp Urol. 2014 Apr 5;2(1):62-70. eCollection 2014. Am J Clin Exp Urol. 2014. PMID: 25374906 Free PMC article. Review.
-
The role of the androgen receptor in the development of prostatic hyperplasia and prostate cancer.Mol Cell Biochem. 2003 Nov;253(1-2):89-101. doi: 10.1023/a:1026057402945. Mol Cell Biochem. 2003. PMID: 14619959 Review.
Cited by
-
Nuclear transport signals control cellular localization and function of androgen receptor cofactor p44/WDR77.PLoS One. 2011;6(7):e22395. doi: 10.1371/journal.pone.0022395. Epub 2011 Jul 15. PLoS One. 2011. PMID: 21789256 Free PMC article.
-
GLI pathogenesis-related 1 functions as a tumor-suppressor in lung cancer.Mol Cancer. 2016 Mar 18;15:25. doi: 10.1186/s12943-016-0508-4. Mol Cancer. 2016. PMID: 26988096 Free PMC article.
-
Protein arginine methyltransferase 5 functions in opposite ways in the cytoplasm and nucleus of prostate cancer cells.PLoS One. 2012;7(8):e44033. doi: 10.1371/journal.pone.0044033. Epub 2012 Aug 27. PLoS One. 2012. PMID: 22952863 Free PMC article.
-
The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond.Cell Mol Life Sci. 2015 Jun;72(11):2041-59. doi: 10.1007/s00018-015-1847-9. Epub 2015 Feb 7. Cell Mol Life Sci. 2015. PMID: 25662273 Free PMC article. Review.
-
Loss of the androgen receptor cofactor p44/WDR77 induces astrogliosis.Mol Cell Biol. 2012 Sep;32(17):3500-12. doi: 10.1128/MCB.00298-12. Epub 2012 Jul 2. Mol Cell Biol. 2012. PMID: 22751923 Free PMC article.
References
-
- Cunha GR, Chung LW 1981 Stromal-epithelial interactions–I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem 14:1317–1324 - PubMed
-
- Donjacour AA, Cunha GR 1993 Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology 132:2342–2350 - PubMed
-
- Rittenhouse HG, Finlay JA, Mikolajczyk SD, Partin AW 1998 Human Kallikrein 2 (hK2) and prostate-specific antigen (PSA): two closely related, but distinct, kallikreins in the prostate. Crit Rev Clin Lab Sci 35:275–368 - PubMed
-
- Isaacs JT, Coffey DS 1989 Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl 2:33–50 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

