Chikungunya virus arthritis in adult wild-type mice
- PMID: 20519386
- PMCID: PMC2916516
- DOI: 10.1128/JVI.02603-09
Chikungunya virus arthritis in adult wild-type mice
Abstract
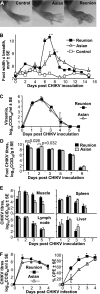
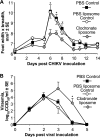
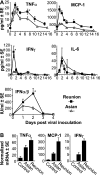
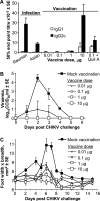
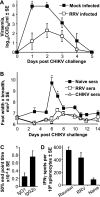
Chikungunya virus is a mosquito-borne arthrogenic alphavirus that has recently reemerged to produce the largest epidemic ever documented for this virus. Here we describe a new adult wild-type mouse model of chikungunya virus arthritis, which recapitulates the self-limiting arthritis, tenosynovitis, and myositis seen in humans. Rheumatic disease was associated with a prolific infiltrate of monocytes, macrophages, and NK cells and the production of monocyte chemoattractant protein 1 (MCP-1), tumor necrosis factor alpha (TNF-alpha), and gamma interferon (IFN-gamma). Infection with a virus isolate from the recent Reunion Island epidemic induced significantly more mononuclear infiltrates, proinflammatory mediators, and foot swelling than did an Asian isolate from the 1960s. Primary mouse macrophages were shown to be productively infected with chikungunya virus; however, the depletion of macrophages ameliorated rheumatic disease and prolonged the viremia. Only 1 microg of an unadjuvanted, inactivated, whole-virus vaccine derived from the Asian isolate completely protected against viremia and arthritis induced by the Reunion Island isolate, illustrating that protection is not strain specific and that low levels of immunity are sufficient to mediate protection. IFN-alpha treatment was able to prevent arthritis only if given before infection, suggesting that IFN-alpha is not a viable therapy. Prior infection with Ross River virus, a related arthrogenic alphavirus, and anti-Ross River virus antibodies protected mice against chikungunya virus disease, suggesting that individuals previously exposed to Ross River virus should be protected from chikungunya virus disease. This new mouse model of chikungunya virus disease thus provides insights into pathogenesis and a simple and convenient system to test potential new interventions.
Figures

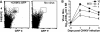
References
-
- Aaskov, J., L. Williams, and S. Yu. 1997. A candidate Ross River virus vaccine: preclinical evaluation. Vaccine 15:1396-1404. - PubMed
-
- Alsharifi, M., M. Lobigs, M. M. Simon, A. Kersten, K. Muller, A. Koskinen, E. Lee, and A. Mullbacher. 2006. NK cell-mediated immunopathology during an acute viral infection of the CNS. Eur. J. Immunol. 36:887-896. - PubMed
-
- Antalis, T. M., M. La Linn, K. Donnan, L. Mateo, J. Gardner, J. L. Dickinson, K. Buttigieg, and A. Suhrbier. 1998. The serine proteinase inhibitor (serpin) plasminogen activation inhibitor type 2 protects against viral cytopathic effects by constitutive interferon alpha/beta priming. J. Exp. Med. 187:1799-1811. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

