Sphingosine 1-phosphate-metabolizing enzymes control influenza virus propagation and viral cytopathogenicity
- PMID: 20519401
- PMCID: PMC2916542
- DOI: 10.1128/JVI.00510-10
Sphingosine 1-phosphate-metabolizing enzymes control influenza virus propagation and viral cytopathogenicity
Abstract
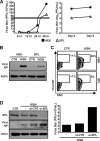
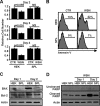
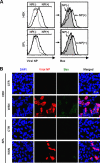
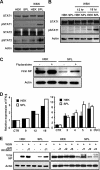
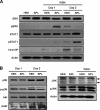
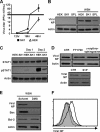
Sphingosine 1-phosphate (S1P)-metabolizing enzymes regulate the level of sphingolipids and have important biological functions. However, the effects of S1P-metabolizing enzymes on host defense against invading viruses remain unknown. In this study, we investigated the role of S1P-metabolizing enzymes in modulating cellular responses to influenza virus infection. Overexpression of S1P lyase (SPL), which induces the degradation of S1P, interfered with the amplification of infectious influenza virus. Accordingly, SPL-overexpressing cells were much more resistant than control cells to the cytopathic effects caused by influenza virus infection. SPL-mediated inhibition of virus-induced cell death was supported by impairment of the upregulation of the proapoptotic protein Bax, a critical factor for influenza virus cytopathogenicity. Importantly, influenza virus infection of SPL-overexpressing cells induced rapid activation of extracellular signal-regulated kinase (ERK) and STAT1 but not of p38 mitogen-activated protein kinase (MAPK), Akt, or c-Jun N-terminal kinase (JNK). Blockade of STAT1 expression or inhibition of Janus kinase (JAK) activity elevated the level of influenza virus replication in the cells, indicating that SPL protects cells from influenza virus via the activation of JAK/STAT signaling. In contrast to that of SPL, the overexpression of S1P-producing sphingosine kinase 1 heightened the cells' susceptibility to influenza virus infection, an effect that was reversed by the inhibition of its kinase activity, representing opposed enzymatic activity. These findings indicate that the modulation of S1P-metabolizing enzymes is crucial for controlling the host defense against infection with influenza virus. Thus, S1P-metabolizing enzymes are novel potential targets for the treatment of diseases caused by influenza virus infection.
Figures
Similar articles
-
Defining the kinetic effects of infection with influenza virus A/PR8/34 (H1N1) on sphingosine-1-phosphate signaling in mice by targeted LC/MS.Sci Rep. 2021 Oct 11;11(1):20161. doi: 10.1038/s41598-021-99765-0. Sci Rep. 2021. PMID: 34635791 Free PMC article.
-
Emerging Connections of S1P-Metabolizing Enzymes with Host Defense and Immunity During Virus Infections.Viruses. 2019 Nov 27;11(12):1097. doi: 10.3390/v11121097. Viruses. 2019. PMID: 31783527 Free PMC article. Review.
-
Sphingosine 1-Phosphate Lyase Enhances the Activation of IKKε To Promote Type I IFN-Mediated Innate Immune Responses to Influenza A Virus Infection.J Immunol. 2017 Jul 15;199(2):677-687. doi: 10.4049/jimmunol.1601959. Epub 2017 Jun 9. J Immunol. 2017. PMID: 28600291 Free PMC article.
-
Sphingosine-1-phosphate lyase is involved in the differentiation of F9 embryonal carcinoma cells to primitive endoderm.J Biol Chem. 2003 Apr 18;278(16):14578-85. doi: 10.1074/jbc.M211416200. Epub 2003 Feb 12. J Biol Chem. 2003. PMID: 12584204
-
Epigenetic regulation of pro-inflammatory cytokine secretion by sphingosine 1-phosphate (S1P) in acute lung injury: Role of S1P lyase.Adv Biol Regul. 2017 Jan;63:156-166. doi: 10.1016/j.jbior.2016.09.007. Epub 2016 Sep 29. Adv Biol Regul. 2017. PMID: 27720306 Free PMC article. Review.
Cited by
-
Fifty years of lyase and a moment of truth: sphingosine phosphate lyase from discovery to disease.J Lipid Res. 2019 Mar;60(3):456-463. doi: 10.1194/jlr.S091181. Epub 2019 Jan 11. J Lipid Res. 2019. PMID: 30635364 Free PMC article. Review.
-
The Sphingolipid Inhibitors Ceranib-2 and SKI-II Reduce Measles Virus Replication in Primary Human Lymphocytes: Effects on mTORC1 Downstream Signaling.Front Physiol. 2022 Mar 17;13:856143. doi: 10.3389/fphys.2022.856143. eCollection 2022. Front Physiol. 2022. PMID: 35370781 Free PMC article.
-
Sphingosine 1-phosphate signaling impacts lymphocyte migration, inflammation and infection.Pathog Dis. 2016 Aug;74(6):ftw063. doi: 10.1093/femspd/ftw063. Epub 2016 Jun 27. Pathog Dis. 2016. PMID: 27354294 Free PMC article. Review.
-
Influenza A virus NS1 induces degradation of sphingosine 1-phosphate lyase to obstruct the host innate immune response.Virology. 2021 Jun;558:67-75. doi: 10.1016/j.virol.2021.02.006. Epub 2021 Mar 13. Virology. 2021. PMID: 33730651 Free PMC article.
-
Epithelial-to-mesenchymal transition and live cell extrusion contribute to measles virus release from human airway epithelia.J Virol. 2025 Feb 25;99(2):e0122024. doi: 10.1128/jvi.01220-24. Epub 2025 Jan 10. J Virol. 2025. PMID: 39791903 Free PMC article.
References
-
- Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. - PubMed
-
- Chalfant, C. E., and S. Spiegel. 2005. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J. Cell Sci. 118:4605-4612. - PubMed
-
- Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. - PubMed
-
- Colié, S., P. P. Van Veldhoven, B. Kedjouar, C. Bedia, V. Albinet, S. C. Sorli, V. Garcia, M. Djavaheri-Mergny, C. Bauvy, P. Codogno, T. Levade, and N. Andrieu-Abadie. 2009. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 69:9346-9353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

