Equol: history, chemistry, and formation
- PMID: 20519412
- PMCID: PMC2884333
- DOI: 10.3945/jn.109.119776
Equol: history, chemistry, and formation
Abstract
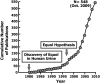
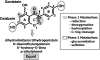
Equol, first isolated from equine urine in 1932 and identified 50 years later in human urine as a metabolite of the soy isoflavones, daidzin and daidzein, is produced by intestinal bacteria in some, but not all, adults. This observation led to the term equol-producers to define those adults that could make equol in response to consuming soy isoflavones and the hypothesis that the health benefits of soy-based diets may be greater in equol-producers than in equol nonproducers. By virtue of a chiral center, equol occurs as a diastereoisomer and intestinal bacteria are enantiospecific in synthesizing exclusively the S-(-)equol enantiomer, an enantiomer that has selective affinity for the estrogen receptor-beta. Both enantiomers are of interest from a clinical and pharmacological perspective and are currently being developed as nutraceutical and pharmacological agents. The wide range of biological activities these enantiomers possess warrants their investigation for the treatment of a number of hormone-related conditions involving estrogen-dependent and androgen-related conditions. The following review describes the history, chemistry, and factors governing the intestinal bacterial formation of equol.
Figures

Similar articles
-
S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora.Am J Clin Nutr. 2005 May;81(5):1072-9. doi: 10.1093/ajcn/81.5.1072. Am J Clin Nutr. 2005. PMID: 15883431 Clinical Trial.
-
Equol: pharmacokinetics and biological actions.J Nutr. 2010 Jul;140(7):1363S-8S. doi: 10.3945/jn.109.119784. Epub 2010 Jun 2. J Nutr. 2010. PMID: 20519411 Free PMC article. Review.
-
The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers.Am J Clin Nutr. 2009 Oct;90(4):1029-37. doi: 10.3945/ajcn.2009.27981. Epub 2009 Aug 26. Am J Clin Nutr. 2009. PMID: 19710188 Free PMC article. Clinical Trial.
-
Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health.Mol Nutr Food Res. 2007 Jul;51(7):765-81. doi: 10.1002/mnfr.200600262. Mol Nutr Food Res. 2007. PMID: 17579894 Review.
-
The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones.J Nutr. 2002 Dec;132(12):3577-84. doi: 10.1093/jn/132.12.3577. J Nutr. 2002. PMID: 12468591 Review.
Cited by
-
Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens.Appl Environ Microbiol. 2013 Jun;79(11):3494-502. doi: 10.1128/AEM.03693-12. Epub 2013 Mar 29. Appl Environ Microbiol. 2013. PMID: 23542626 Free PMC article.
-
Diversity, stability and resilience of the human gut microbiota.Nature. 2012 Sep 13;489(7415):220-30. doi: 10.1038/nature11550. Nature. 2012. PMID: 22972295 Free PMC article. Review.
-
Can soy isoflavones in combination with soy protein change serum concentration of adiponectin and resistin? A systematic review and meta-analysis on randomized clinical trials.Food Sci Nutr. 2022 Sep 15;10(12):4126-4138. doi: 10.1002/fsn3.3038. eCollection 2022 Dec. Food Sci Nutr. 2022. PMID: 36514764 Free PMC article. Review.
-
Microbial Metabolites Determine Host Health and the Status of Some Diseases.Int J Mol Sci. 2019 Oct 24;20(21):5296. doi: 10.3390/ijms20215296. Int J Mol Sci. 2019. PMID: 31653062 Free PMC article. Review.
-
Esterases From Bifidobacteria Exhibit the Conversion of Albiflorin in Gut Microbiota.Front Microbiol. 2022 Apr 6;13:880118. doi: 10.3389/fmicb.2022.880118. eCollection 2022. Front Microbiol. 2022. PMID: 35464989 Free PMC article.
References
-
- Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust J Agric Res. 1946;22:131–8. - PubMed
-
- Lightfoot RJ, Croker KP, Neil HG. Failure of sperm transport in relation to ewe infertility following prolonged grazing on oestrogenic pastures. Aust J Agric Res. 1968;18:755–65.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

