Equol: history, chemistry, and formation
- PMID: 20519412
- PMCID: PMC2884333
- DOI: 10.3945/jn.109.119776
Equol: history, chemistry, and formation
Abstract
Equol, first isolated from equine urine in 1932 and identified 50 years later in human urine as a metabolite of the soy isoflavones, daidzin and daidzein, is produced by intestinal bacteria in some, but not all, adults. This observation led to the term equol-producers to define those adults that could make equol in response to consuming soy isoflavones and the hypothesis that the health benefits of soy-based diets may be greater in equol-producers than in equol nonproducers. By virtue of a chiral center, equol occurs as a diastereoisomer and intestinal bacteria are enantiospecific in synthesizing exclusively the S-(-)equol enantiomer, an enantiomer that has selective affinity for the estrogen receptor-beta. Both enantiomers are of interest from a clinical and pharmacological perspective and are currently being developed as nutraceutical and pharmacological agents. The wide range of biological activities these enantiomers possess warrants their investigation for the treatment of a number of hormone-related conditions involving estrogen-dependent and androgen-related conditions. The following review describes the history, chemistry, and factors governing the intestinal bacterial formation of equol.
Figures
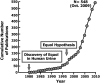
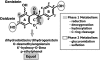

References
-
- Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust J Agric Res. 1946;22:131–8. - PubMed
-
- Lightfoot RJ, Croker KP, Neil HG. Failure of sperm transport in relation to ewe infertility following prolonged grazing on oestrogenic pastures. Aust J Agric Res. 1968;18:755–65.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

