Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis
- PMID: 20519446
- PMCID: PMC2944320
- DOI: 10.1152/ajpcell.00535.2009
Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis
Abstract
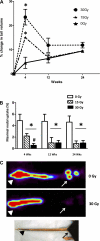
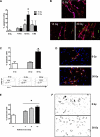
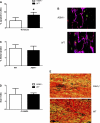
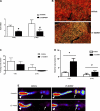
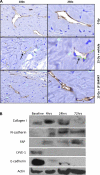
Although radiation therapy is a major risk factor for the development of lymphedema following lymphadenectomy, the mechanisms responsible for this effect remain unknown. The purpose of this study was therefore to determine the effects of radiation on lymphatic endothelial cells (LECs) and lymphatic function. The tails of wild-type or acid sphingomyelinase (ASM)-deficient mice were treated with 0, 15, or 30 Gy of radiation and then analyzed for LEC apoptosis and lymphatic function at various time points. To analyze the effects of radiation fibrosis on lymphatic function, we determined the effects of transforming growth factor (TGF)-beta1 blockade after radiation in vivo. Finally, we determined the effects of radiation and exogenous TGF-beta1 on LECs in vitro. Radiation caused mild edema that resolved after 12-24 wk. Interestingly, despite resolution of tail edema, irradiated animals displayed persistent lymphatic dysfunction. Radiation caused loss of capillary lymphatics and was associated with a dose-dependent increase in LEC apoptosis. ASM-/- mice had significantly less LEC apoptosis; however, this finding did not translate to improved lymphatic function at later time points. Short-term blockade of TGF-beta1 function after radiation markedly decreased tissue fibrosis and significantly improved lymphatic function but did not alter LEC apoptosis. Radiation therapy decreases lymphatic reserve by causing depletion of lymphatic vessels and LECs as well as promoting soft tissue fibrosis. Short-term inhibition of TGF-beta1 activity following radiation improves lymphatic function and is associated with decreased soft tissue fibrosis. ASM deficiency confers LEC protection from radiation-induced apoptosis but does not prevent lymphatic dysfunction.
Figures




References
-
- Abu-Rustum NR, Alektiar K, Iasonos A, Lev G, Sonoda Y, Aghajanian C, Chi DS, Barakat RR. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol 103: 714–718, 2006 - PubMed
-
- Avraham T, Clavin NW, Daluvoy SV, Fernandez J, Soares MA, Cordeiro AP, Mehrara BJ. Fibrosis is a key inhibitor of lymphatic regeneration. Plast Reconstr Surg 124: 438–450, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

