Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue
- PMID: 20519448
- PMCID: PMC2944322
- DOI: 10.1152/ajpcell.00065.2010
Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue
Abstract
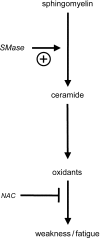
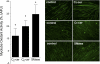
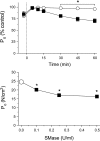
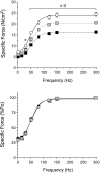
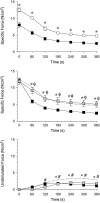
Sphingomyelinase (SMase) hydrolyzes membrane sphingomyelin into ceramide, which increases oxidants in nonmuscle cells. Serum SMase activity is elevated in sepsis and heart failure, conditions where muscle oxidants are increased, maximal muscle force is diminished, and fatigue is accelerated. We tested the hypotheses that exogenous SMase and accumulation of ceramide in muscle increases oxidants in muscle cells, depresses specific force of unfatigued muscle, and accelerates the fatigue process. We also anticipated that the antioxidant N-acetylcysteine (NAC) would prevent SMase effects on muscle function. We studied the responses of C2C12 myotubes and mouse diaphragm to SMase treatment in vitro. We observed that SMase caused a 2.8-fold increase in total ceramide levels in myotubes. Exogenous ceramide and SMase elevated oxidant activity in C2C12 myotubes by 15-35% (P < 0.05) and in diaphragm muscle fiber bundles by 58-120% (P < 0.05). The SMase-induced increase in diaphragm oxidant activity was prevented by NAC. Exogenous ceramide depressed diaphragm force by 55% (P < 0.05), while SMase depressed maximal force by 30% (P < 0.05) and accelerated fatigue--effects opposed by treatment with NAC. In conclusion, our findings suggest that SMase stimulates a ceramide-oxidant signaling pathway that results in muscle weakness and fatigue.
Figures
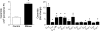


Comment in
-
A novel role for sphingolipid metabolism in oxidant-mediated skeletal muscle fatigue. Focus on "Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue".Am J Physiol Cell Physiol. 2010 Sep;299(3):C549-51. doi: 10.1152/ajpcell.00236.2010. Epub 2010 Jun 23. Am J Physiol Cell Physiol. 2010. PMID: 20573998 No abstract available.
Similar articles
-
Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers.J Appl Physiol (1985). 2012 May;112(9):1538-45. doi: 10.1152/japplphysiol.01269.2011. Epub 2012 Feb 23. J Appl Physiol (1985). 2012. PMID: 22362402 Free PMC article.
-
A novel role for sphingolipid metabolism in oxidant-mediated skeletal muscle fatigue. Focus on "Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue".Am J Physiol Cell Physiol. 2010 Sep;299(3):C549-51. doi: 10.1152/ajpcell.00236.2010. Epub 2010 Jun 23. Am J Physiol Cell Physiol. 2010. PMID: 20573998 No abstract available.
-
Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content.Eur J Heart Fail. 2014 May;16(5):519-25. doi: 10.1002/ejhf.73. Epub 2014 Mar 4. Eur J Heart Fail. 2014. PMID: 24596158 Free PMC article.
-
Neutral sphingomyelinase: past, present and future.Chem Phys Lipids. 1999 Nov;102(1-2):79-96. doi: 10.1016/s0009-3084(99)00077-8. Chem Phys Lipids. 1999. PMID: 11001563 Review.
-
Ceramide and sphingomyelinases in the regulation of stress responses.Chem Phys Lipids. 1999 Nov;102(1-2):141-7. doi: 10.1016/s0009-3084(99)00082-1. Chem Phys Lipids. 1999. PMID: 11001568 Review.
Cited by
-
Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers.J Appl Physiol (1985). 2012 May;112(9):1538-45. doi: 10.1152/japplphysiol.01269.2011. Epub 2012 Feb 23. J Appl Physiol (1985). 2012. PMID: 22362402 Free PMC article.
-
Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males.J Appl Physiol (1985). 2012 Dec 1;113(11):1727-36. doi: 10.1152/japplphysiol.00412.2012. Epub 2012 Oct 4. J Appl Physiol (1985). 2012. PMID: 23042913 Free PMC article.
-
Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology.Heart Fail Rev. 2017 Mar;22(2):191-207. doi: 10.1007/s10741-016-9549-4. Heart Fail Rev. 2017. PMID: 27000754 Free PMC article. Review.
-
Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance.Diabetologia. 2011 Jul;54(7):1596-607. doi: 10.1007/s00125-011-2127-3. Epub 2011 Apr 6. Diabetologia. 2011. PMID: 21468641 Review.
-
Skeletal myofiber VEGF deficiency leads to mitochondrial, structural, and contractile alterations in mouse diaphragm.J Appl Physiol (1985). 2019 Nov 1;127(5):1360-1369. doi: 10.1152/japplphysiol.00779.2018. Epub 2019 Sep 5. J Appl Physiol (1985). 2019. PMID: 31487223 Free PMC article.
References
-
- Arena S, Pattarozzi A, Thellung S, Villa V, Corsaro A, Massa A, Diana F, Spoto G, Forcella S, Damonte G, Filocamo M, Benatti U, Schettini G, Florioa T. Nitric oxide production stimulated by the basic fibroblast growth factor requires the synthesis of ceramide. Ann NY Acad Sci 973: 94–104, 2002 - PubMed
-
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6: 593–597, 1989 - PubMed
-
- Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol 90: 45–54, 2001 - PubMed
-
- Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

