DNA polymerase beta ribonucleotide discrimination: insertion, misinsertion, extension, and coding
- PMID: 20519499
- PMCID: PMC2915682
- DOI: 10.1074/jbc.M110.132407
DNA polymerase beta ribonucleotide discrimination: insertion, misinsertion, extension, and coding
Abstract
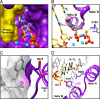
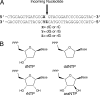
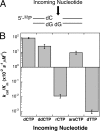
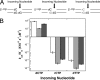
DNA polymerases must select nucleotides that preserve Watson-Crick base pairing rules and choose substrates with the correct (deoxyribose) sugar. Sugar discrimination represents a great challenge because ribonucleotide triphosphates are present at much higher cellular concentrations than their deoxy-counterparts. Although DNA polymerases discriminate against ribonucleotides, many therapeutic nucleotide analogs that target polymerases have sugar modifications, and their efficacy depends on their ability to be incorporated into DNA. Here, we investigate the ability of DNA polymerase beta to utilize nucleotides with modified sugars. DNA polymerase beta readily inserts dideoxynucleoside triphosphates but inserts ribonucleotides nearly 4 orders of magnitude less efficiently than natural deoxynucleotides. The efficiency of ribonucleotide insertion is similar to that reported for other DNA polymerases. The poor polymerase-dependent insertion represents a key step in discriminating against ribonucleotides because, once inserted, a ribonucleotide is easily extended. Likewise, a templating ribonucleotide has little effect on insertion efficiency or fidelity. In contrast to insertion and extension of a ribonucleotide, the chemotherapeutic drug arabinofuranosylcytosine triphosphate is efficiently inserted but poorly extended. These results suggest that the sugar pucker at the primer terminus plays a crucial role in DNA synthesis; a 3'-endo sugar pucker facilitates nucleotide insertion, whereas a 2'-endo conformation inhibits insertion.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

