Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling
- PMID: 20519507
- PMCID: PMC2915703
- DOI: 10.1074/jbc.M109.091256
Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling
Abstract
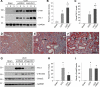
Plasminogen activator inhibitor-1 (PAI-1) is a multifunctional glycoprotein that plays a critical role in the pathogenesis of chronic kidney and cardiovascular diseases. Although transforming growth factor (TGF)-beta1 is a known inducer of PAI-1, how it controls PAI-1 expression remains enigmatic. Here we investigated the mechanism underlying TGF-beta1 regulation of PAI-1 in kidney tubular epithelial cells (HKC-8). Surprisingly, overexpression of Smad2 or Smad3 in HKC-8 cells blocked PAI-1 induction by TGF-beta1, whereas knockdown of them sensitized the cells to TGF-beta1 stimulation, suggesting that Smad signaling is not responsible for PAI-1 induction. Blockade of several TGF-beta1 downstream pathways such as p38 MAPK or JNK, but not phosphatidylinositol 3-kinase/Akt and ERK1/2, only partially inhibited PAI-1 expression. TGF-beta1 stimulated beta-catenin activation in tubular epithelial cells, and ectopic expression of beta-catenin induced PAI-1 expression, whereas inhibition of beta-catenin abolished its induction. A functional T cell factor/lymphoid enhancer-binding factor-binding site was identified in the promoter region of the PAI-1 gene, which interacted with T cell factor upon beta-catenin activation. Deletion or site-directed mutation of this site abolished PAI-1 response to beta-catenin or TGF-beta1 stimulation. Similarly, ectopic expression of Wnt1 also activated PAI-1 expression and promoter activity. In vivo, PAI-1 was induced in kidney tubular epithelia in obstructive nephropathy. Delivery of Wnt1 gene activated beta-catenin and promoted PAI-1 expression after obstructive injury, whereas blockade of Wnt/beta-catenin signaling by Dickkopf-1 gene inhibited PAI-1 induction. Collectively, these studies identify PAI-1 as a direct downstream target of Wnt/beta-catenin signaling and demonstrate that PAI-1 induction could play a role in mediating the fibrogenic action of this signaling.
Figures


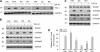


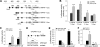



Similar articles
-
Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria.Kidney Int. 2011 Dec;80(11):1159-1169. doi: 10.1038/ki.2011.255. Epub 2011 Aug 10. Kidney Int. 2011. PMID: 21832980 Free PMC article.
-
Transforming growth factor-beta1-dependent activation of Smad2/3 and up-regulation of PAI-1 expression is negatively regulated by Src in SKOV-3 human ovarian cancer cells.J Cell Biochem. 2004 Oct 15;93(3):437-53. doi: 10.1002/jcb.20160. J Cell Biochem. 2004. PMID: 15372629
-
TGF-beta1-induced plasminogen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60(c-src)/EGFR(Y845) and Rho/ROCK signaling.J Mol Cell Cardiol. 2008 Mar;44(3):527-38. doi: 10.1016/j.yjmcc.2007.12.006. Epub 2008 Jan 3. J Mol Cell Cardiol. 2008. PMID: 18255094 Free PMC article.
-
Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells.Thromb Haemost. 2008 Dec;100(6):976-83. Thromb Haemost. 2008. PMID: 19132220 Free PMC article. Review.
-
The TGF-β1/p53/PAI-1 Signaling Axis in Vascular Senescence: Role of Caveolin-1.Biomolecules. 2019 Aug 3;9(8):341. doi: 10.3390/biom9080341. Biomolecules. 2019. PMID: 31382626 Free PMC article. Review.
Cited by
-
Activation of Wnt11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells.J Biol Chem. 2012 Jun 15;287(25):21290-302. doi: 10.1074/jbc.M112.357202. Epub 2012 May 3. J Biol Chem. 2012. PMID: 22556418 Free PMC article.
-
Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells.Arthritis Rheum. 2012 Aug;64(8):2734-45. doi: 10.1002/art.34424. Arthritis Rheum. 2012. PMID: 22328118 Free PMC article.
-
Novel mechanism of resistance to targeted therapies in lung cancer.Mol Cell Oncol. 2018 Dec 30;6(1):1551015. doi: 10.1080/23723556.2018.1551015. eCollection 2019. Mol Cell Oncol. 2018. PMID: 30788420 Free PMC article.
-
Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria.Kidney Int. 2011 Dec;80(11):1159-1169. doi: 10.1038/ki.2011.255. Epub 2011 Aug 10. Kidney Int. 2011. PMID: 21832980 Free PMC article.
-
Disruption of the Dapper3 gene aggravates ureteral obstruction-mediated renal fibrosis by amplifying Wnt/β-catenin signaling.J Biol Chem. 2013 May 24;288(21):15006-14. doi: 10.1074/jbc.M113.458448. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580654 Free PMC article.
References
-
- Eddy A. A., Fogo A. B. (2006) J. Am. Soc. Nephrol. 17, 2999–3012 - PubMed
-
- Nagamine Y. (2008) Thromb. Haemost. 100, 1007–1013 - PubMed
-
- Hamano K., Iwano M., Akai Y., Sato H., Kubo A., Nishitani Y., Uyama H., Yoshida Y., Miyazaki M., Shiiki H., Kohno S., Dohi K. (2002) Am. J. Kidney Dis. 39, 695–705 - PubMed
-
- Paueksakon P., Revelo M. P., Ma L. J., Marcantoni C., Fogo A. B. (2002) Kidney Int. 61, 2142–2148 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

