Protein tyrosine phosphatases are regulated by mononuclear iron dicitrate
- PMID: 20519508
- PMCID: PMC2915698
- DOI: 10.1074/jbc.M110.107037
Protein tyrosine phosphatases are regulated by mononuclear iron dicitrate
Abstract
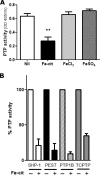
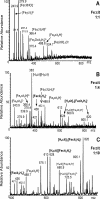
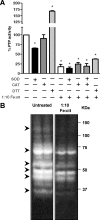
The involvement of macrophages (Mvarphis) as host, accessory, and effector cells in the development of infectious diseases, together with their central role in iron homeostasis, place these immune cells as key players in the interface between iron and infection. Having previously shown that the functional expression of NRAMP-1 results in increased protein phosphorylation mediated in part by an iron-dependent inhibition of Mvarphi protein-tyrosine phosphatase (PTP) activity, we sought to study the mechanism(s) underlying this specific event. Herein we have identified the mononuclear dicitrate iron complex [Fe(cit)(2)H(4-x)]((1+x)-) as the species responsible for the specific inhibition of Mvarphi PTP activity. By using biochemical and computational approaches, we show that [Fe(cit)(2)](5-) targets the catalytic pocket of the PTP SHP-1, competitively inhibiting its interaction with an incoming phosphosubstrate. In vitro and in vivo inhibition of PTP activity by iron-citrate results in protein hyperphosphorylation and enhanced MAPK signaling in response to LPS stimulation. We propose that iron-citrate-mediated PTP inhibition represents a novel and biologically relevant regulatory mechanism of signal transduction.
Figures



References
-
- Johnson L. N., Lewis R. J. (2001) Chem. Rev. 101, 2209–2242 - PubMed
-
- Mustelin T., Vang T., Bottini N. (2005) Nat. Rev. Immunol. 5, 43–57 - PubMed
-
- Bilwes A. M., den Hertog J., Hunter T., Noel J. P. (1996) Nature 382, 555–559 - PubMed
-
- Tonks N. K. (2005) Cell 121, 667–670 - PubMed
-
- Alonso A., Sasin J., Bottini N., Friedberg I., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. (2004) Cell 117, 699–711 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

