Interleukin-27 induces a STAT1/3- and NF-kappaB-dependent proinflammatory cytokine profile in human monocytes
- PMID: 20519510
- PMCID: PMC2915676
- DOI: 10.1074/jbc.M110.112599
Interleukin-27 induces a STAT1/3- and NF-kappaB-dependent proinflammatory cytokine profile in human monocytes
Erratum in
- J Biol Chem. 2012 Mar 9;287(11):8661
Abstract
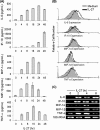
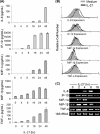
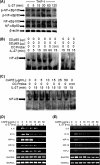
IL-27 is a heterodimeric cytokine bridging innate and adaptive immunity by playing a role in the activation of naive T cells and in development of Th1 cells. Additionally, recent evidence supports a role for IL-27 in the activation of monocytic cells. Both pro-inflammatory and anti-inflammatory activities have been attributed to IL-27; however, the role played by IL-27 in the activation of human monocytic cells in terms of cytokine production has not been well described. Our results show that IL-27 is a strong inducer of proinflammatory cytokine and chemokine expression, including enhancement of IL-6, IP-10, MIP-1alpha, MIP-1beta, and TNF-alpha expression in human primary monocytes. Furthermore, we observed that IL-27-induced cytokine and chemokine production was mediated by STAT1, STAT3, and NF-kappaB activation. Understanding how IL-27 exerts its effects on monocytic cells will identify important molecular mechanisms in the regulation of immune responses, particularly in the modulation of monocyte activation.
Figures


Similar articles
-
Acute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytes.Alcohol Clin Exp Res. 2008 Sep;32(9):1565-73. doi: 10.1111/j.1530-0277.2008.00726.x. Epub 2008 Jul 9. Alcohol Clin Exp Res. 2008. PMID: 18616672 Free PMC article.
-
TWEAK/Fn14 promotes pro-inflammatory cytokine secretion in hepatic stellate cells via NF-κB/STAT3 pathways.Mol Immunol. 2017 Jul;87:67-75. doi: 10.1016/j.molimm.2017.04.003. Epub 2017 Apr 12. Mol Immunol. 2017. PMID: 28411440
-
The NF-kappaB, p38 MAPK and STAT1 pathways differentially regulate the dsRNA-mediated innate immune responses of epidermal keratinocytes.Int Immunol. 2008 Jul;20(7):901-9. doi: 10.1093/intimm/dxn048. Epub 2008 May 19. Int Immunol. 2008. PMID: 18492658
-
IL-12, IL-23, and IL-27 enhance human beta-defensin-2 production in human keratinocytes.Eur J Immunol. 2008 May;38(5):1287-96. doi: 10.1002/eji.200738051. Eur J Immunol. 2008. PMID: 18389480
-
Essential involvement of cross-talk between IFN-gamma and TNF-alpha in CXCL10 production in human THP-1 monocytes.J Cell Physiol. 2009 Sep;220(3):690-7. doi: 10.1002/jcp.21815. J Cell Physiol. 2009. PMID: 19472212
Cited by
-
Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis.Curr Opin Allergy Clin Immunol. 2012 Dec;12(6):616-22. doi: 10.1097/ACI.0b013e328358cc0b. Curr Opin Allergy Clin Immunol. 2012. PMID: 23026768 Free PMC article. Review.
-
IL-12 Family Cytokines in Cancer and Immunotherapy.Cancers (Basel). 2021 Jan 6;13(2):167. doi: 10.3390/cancers13020167. Cancers (Basel). 2021. PMID: 33418929 Free PMC article. Review.
-
Interleukin-27 Is a Potential Rescue Therapy for Acute Severe Colitis Through Interleukin-10-Dependent, T-Cell-Independent Attenuation of Colonic Mucosal Innate Immune Responses.Inflamm Bowel Dis. 2017 Nov;23(11):1983-1995. doi: 10.1097/MIB.0000000000001274. Inflamm Bowel Dis. 2017. PMID: 29019857 Free PMC article.
-
Systemic IL-27 administration prevents abscess formation and osteolysis via local neutrophil recruitment and activation.Bone Res. 2022 Aug 26;10(1):56. doi: 10.1038/s41413-022-00228-7. Bone Res. 2022. PMID: 36028492 Free PMC article.
-
TLR7 Ligation Inhibits TLR8 Responsiveness in IL-27-Primed Human THP-1 Monocytes and Macrophages.J Innate Immun. 2021;13(6):345-358. doi: 10.1159/000515738. Epub 2021 May 31. J Innate Immun. 2021. PMID: 34058746 Free PMC article.
References
-
- Batten M., Ghilardi N. (2007) J. Mol. Med. 85, 661–672 - PubMed
-
- Kastelein R. A., Hunter C. A., Cua D. J. (2007) Annu. Rev. Immunol. 25, 221–242 - PubMed
-
- Villarino A. V., Huang E., Hunter C. A. (2004) J. Immunol. 173, 715–720 - PubMed
-
- Pflanz S., Timans J. C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., Blumenschein W. M., Mattson J. D., Wagner J. L., To W., Zurawski S., McClanahan T. K., Gorman D. M., Bazan J. F., de Waal M. R., Rennick D., Kastelein R. A. (2002) Immunity 16, 779–790 - PubMed
-
- Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J. F., Phillips J. H., McClanahan T. K., de Waal M. R., Kastelein R. A. (2004) J. Immunol. 172, 2225–2231 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

