Spinal cord injury immediately changes the state of the brain
- PMID: 20519527
- PMCID: PMC3842476
- DOI: 10.1523/JNEUROSCI.0379-10.2010
Spinal cord injury immediately changes the state of the brain
Abstract
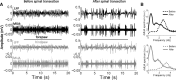
Spinal cord injury can produce extensive long-term reorganization of the cerebral cortex. Little is known, however, about the sequence of cortical events starting immediately after the lesion. Here we show that a complete thoracic transection of the spinal cord produces immediate functional reorganization in the primary somatosensory cortex of anesthetized rats. Besides the obvious loss of cortical responses to hindpaw stimuli (below the level of the lesion), cortical responses evoked by forepaw stimuli (above the level of the lesion) markedly increase. Importantly, these increased responses correlate with a slower and overall more silent cortical spontaneous activity, representing a switch to a network state of slow-wave activity similar to that observed during slow-wave sleep. The same immediate cortical changes are observed after reversible pharmacological block of spinal cord conduction, but not after sham. We conclude that the deafferentation due to spinal cord injury can immediately (within minutes) change the state of large cortical networks, and that this state change plays a critical role in the early cortical reorganization after spinal cord injury.
Figures



References
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Arthurs OJ, Boniface SJ. What aspect of the fMRI BOLD signal best reflects the underlying electrophysiology in human somatosensory cortex? Clin Neurophysiol. 2003;114:1203–1209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
