Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons
- PMID: 20519533
- PMCID: PMC2901931
- DOI: 10.1523/JNEUROSCI.0389-10.2010
Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons
Erratum in
- J Neurosci. 2012 Apr 18;32(16):5704
Abstract
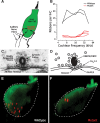
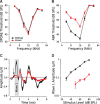
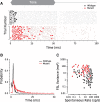
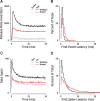
Synaptic ribbons, found at the presynaptic membrane of sensory cells in both ear and eye, have been implicated in the vesicle-pool dynamics of synaptic transmission. To elucidate ribbon function, we characterized the response properties of single auditory nerve fibers in mice lacking Bassoon, a scaffolding protein involved in anchoring ribbons to the membrane. In bassoon mutants, immunohistochemistry showed that fewer than 3% of the hair cells' afferent synapses retained anchored ribbons. Auditory nerve fibers from mutants had normal threshold, dynamic range, and postonset adaptation in response to tone bursts, and they were able to phase lock with normal precision to amplitude-modulated tones. However, spontaneous and sound-evoked discharge rates were reduced, and the reliability of spikes, particularly at stimulus onset, was significantly degraded as shown by an increased variance of first-spike latencies. Modeling based on in vitro studies of normal and mutant hair cells links these findings to reduced release rates at the synapse. The degradation of response reliability in these mutants suggests that the ribbon and/or Bassoon normally facilitate high rates of exocytosis and that its absence significantly compromises the temporal resolving power of the auditory system.
Figures



References
-
- Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fässler R, Richter K, Boeckers TM, Potschka H, Brandt C, Löscher W, Grimberg D, Dresbach T, Hempelmann A, Hassan H, Balschun D, Frey JU, Brandstätter JH, Garner CC, et al. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787–800. - PubMed
-
- Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex. I. Heterogeneity of responses. J Neurophysiol. 1997;78:1222–1236. - PubMed
-
- Bernstein LR, Trahiotis C. Enhancing sensitivity to interaural delays at high frequencies by using “transposed stimuli.”. J Acoust Soc Am. 2002;112:1026–1036. - PubMed
-
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
