microRNA-dependent modulation of histone acetylation in Waldenstrom macroglobulinemia
- PMID: 20519629
- PMCID: PMC2938840
- DOI: 10.1182/blood-2010-01-265686
microRNA-dependent modulation of histone acetylation in Waldenstrom macroglobulinemia
Abstract
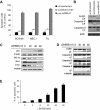
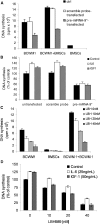
Waldenström macroglobulinemia (WM) cells present with increased expression of microRNA-206 (miRNA-206) and reduced expression of miRNA-9*. Predicted miRNA-206- and -9*-targeted genes include histone deacetylases (HDACs) and histone acetyl transferases (HATs), indicating that these miRNAs may play a role in regulating histone acetylation. We were able to demonstrate that primary WM cells are characterized by unbalanced expression of HDACs and HATs, responsible for decreased acetylated histone-H3 and -H4, and increased HDAC activity. We next examined whether miRNA-206 and -9* modulate the aberrant expression of HDAC and HATs in WM cells leading to increased transcriptional activity. We found that restoring miRNA-9* levels induced toxicity in WM cells, supported by down-modulation of HDAC4 and HDAC5 and up-regulation of acetyl-histone-H3 and -H4. These, together with inhibited HDAC activity, led to induction of apoptosis and autophagy in WM cells. To further confirm that miRNA-9*-dependent modulation of histone acetylation is responsible for induction of WM cytotoxicity, a novel class of HDAC inhibitor (LBH589) was used; we confirmed that inhibition of HDAC activity leads to toxicity in this disease. These findings confirm that histone-modifying genes and HDAC activity are deregulated in WM cells, partially driven by the aberrant expression of miRNA-206 and -9* in the tumor clone.
Figures





Similar articles
-
Using Histone Deacetylase Inhibitors to Analyze the Relevance of HDACs for Translation.Methods Mol Biol. 2017;1510:77-91. doi: 10.1007/978-1-4939-6527-4_6. Methods Mol Biol. 2017. PMID: 27761814
-
microRNA expression in the biology, prognosis, and therapy of Waldenström macroglobulinemia.Blood. 2009 Apr 30;113(18):4391-402. doi: 10.1182/blood-2008-09-178228. Epub 2008 Dec 12. Blood. 2009. PMID: 19074725 Free PMC article.
-
Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes.Cell. 2009 Sep 4;138(5):1019-31. doi: 10.1016/j.cell.2009.06.049. Epub 2009 Aug 20. Cell. 2009. PMID: 19698979 Free PMC article.
-
Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy.Life Sci. 2021 Jul 15;277:119504. doi: 10.1016/j.lfs.2021.119504. Epub 2021 Apr 16. Life Sci. 2021. PMID: 33872660 Review.
-
The role of histone deacetylases (HDACs) in human cancer.Mol Oncol. 2007 Jun;1(1):19-25. doi: 10.1016/j.molonc.2007.01.001. Epub 2007 Mar 7. Mol Oncol. 2007. PMID: 19383284 Free PMC article. Review.
Cited by
-
Causes and consequences of microRNA dysregulation.Cancer J. 2012 May-Jun;18(3):215-22. doi: 10.1097/PPO.0b013e318250c001. Cancer J. 2012. PMID: 22647357 Free PMC article. Review.
-
MicroRNAs in B-cells: from normal differentiation to treatment of malignancies.Oncotarget. 2015 Jan 1;6(1):7-25. doi: 10.18632/oncotarget.3057. Oncotarget. 2015. PMID: 25622103 Free PMC article. Review.
-
Nucleic Acid Biomarkers in Waldenström Macroglobulinemia and IgM-MGUS: Current Insights and Clinical Relevance.Diagnostics (Basel). 2022 Apr 12;12(4):969. doi: 10.3390/diagnostics12040969. Diagnostics (Basel). 2022. PMID: 35454017 Free PMC article. Review.
-
The Non-Coding RNA Landscape of Plasma Cell Dyscrasias.Cancers (Basel). 2020 Jan 30;12(2):320. doi: 10.3390/cancers12020320. Cancers (Basel). 2020. PMID: 32019064 Free PMC article. Review.
-
Hydroxychloroquine facilitates autophagosome formation but not degradation to suppress the proliferation of cervical cancer SiHa cells.Oncol Lett. 2014 Apr;7(4):1057-1062. doi: 10.3892/ol.2014.1879. Epub 2014 Feb 13. Oncol Lett. 2014. PMID: 24944668 Free PMC article.
References
-
- Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003;4(11):679–685. - PubMed
-
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenström's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström's Macroglobulinemia. Semin Oncol. 2003;30(2):110–115. - PubMed
-
- Schop RF, Kuehl WM, Van Wier SA, et al. Waldenström macroglobulinemia neoplastic cells lack immunoglobulin heavy chain locus translocations but have frequent 6q deletions. Blood. 2002;100(8):2996–3001. - PubMed
-
- Esteller M. Epigenetics in Cancer. N Engl J Med. 2008;358(11):1148–1159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

