Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer
- PMID: 20519679
- PMCID: PMC2935316
- DOI: 10.1056/NEJMoa0909638
Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer
Abstract
Background: Chemotherapy regimens that combine anthracyclines and taxanes result in improved disease-free and overall survival among women with operable lymph-node-positive breast cancer. The effectiveness of concurrent versus sequential regimens is not known.
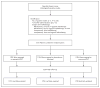
Methods: We randomly assigned 5351 patients with operable, node-positive, early-stage breast cancer to receive four cycles of doxorubicin and cyclophosphamide followed by four cycles of docetaxel (sequential ACT); four cycles of doxorubicin and docetaxel (doxorubicin-docetaxel); or four cycles of doxorubicin, cyclophosphamide, and docetaxel (concurrent ACT). The primary aims were to examine whether concurrent ACT was more effective than sequential ACT and whether the doxorubicin-docetaxel regimen would be as effective as the concurrent-ACT regimen. The secondary aims were to assess toxic effects and to correlate amenorrhea with outcomes in premenopausal women.
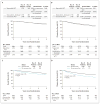
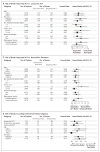
Results: At a median follow-up of 73 months, overall survival was improved in the sequential-ACT group (8-year overall survival, 83%) as compared with the doxorubicin-docetaxel group (overall survival, 79%; hazard ratio for death, 0.83; P=0.03) and the concurrent-ACT group (overall survival, 79%; hazard ratio, 0.86; P=0.09). Disease-free survival was improved in the sequential-ACT group (8-year disease-free survival, 74%) as compared with the doxorubicin-docetaxel group (disease-free survival, 69%; hazard ratio for recurrence, a second malignant condition, or death, 0.80; P=0.001) and the concurrent-ACT group (disease-free survival, 69%; hazard ratio, 0.83; P=0.01). The doxorubicin-docetaxel regimen showed noninferiority to the concurrent-ACT regimen for overall survival (hazard ratio, 0.96; 95% confidence interval, 0.82 to 1.14). Overall survival was improved in patients with amenorrhea for 6 months or more across all treatment groups, independently of estrogen-receptor status.
Conclusions: Sequential ACT improved disease-free survival as compared with doxorubicin-docetaxel or concurrent ACT, and it improved overall survival as compared with doxorubicin-docetaxel. Amenorrhea was associated with improved survival regardless of the treatment and estrogen-receptor status. (ClinicalTrials.gov number, NCT00003782.)
2010 Massachusetts Medical Society
Figures

Comment in
-
Taxane-based chemotherapy for node-positive breast cancer--take-home lessons.N Engl J Med. 2010 Jun 3;362(22):2122-4. doi: 10.1056/NEJMe1003854. N Engl J Med. 2010. PMID: 20519684 No abstract available.
-
Duration of chemotherapy for early stage breast cancer: have we reached the limit? What is the survival impact of amenorrhea?Curr Treat Options Oncol. 2010 Dec;11(3-4):59-62. doi: 10.1007/s11864-010-0123-3. Curr Treat Options Oncol. 2010. PMID: 21061193 No abstract available.
-
Amenorrhea from breast cancer therapy--not a matter of dose.N Engl J Med. 2010 Dec 2;363(23):2268-70. doi: 10.1056/NEJMc1009616. N Engl J Med. 2010. PMID: 21121855 No abstract available.
References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–717. - PubMed
-
- Nabholtz JM, Falkson C, Campos D, et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol. 2003;21:968–75. [Erratum, J Clin Oncol 2003;21:2048.] - PubMed
-
- Mackey JR, Paterson A, Dirix LY, et al. Final results of the phase III randomized trial comparing docetaxel (T), doxorubicin (A) and cyclophosphamide (C) to FAC as first line chemotherapy (CT) for patients (pts) with metastatic breast cancer (MBC) Proc Am Soc Clin Oncol. 2002;21:35a. abstract.
-
- Knobf MT. The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. Oncologist. 2006;11:96–110. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10-CA-69651/CA/NCI NIH HHS/United States
- CA07190/CA/NCI NIH HHS/United States
- U10-CA-37377/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- CA-25224/CA/NCI NIH HHS/United States
- CA-45808/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U10 CA058348/CA/NCI NIH HHS/United States
- U10 CA037377/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA012027/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10-CA-69974/CA/NCI NIH HHS/United States
- CA-58348/CA/NCI NIH HHS/United States
- U10-CA-12027/CA/NCI NIH HHS/United States
- CA2115/CA/NCI NIH HHS/United States
- CA-13612/CA/NCI NIH HHS/United States
- CA-32102/CA/NCI NIH HHS/United States
- U10 CA069651/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA069974/CA/NCI NIH HHS/United States
- U10 CA007190/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
