Cumulus-oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro
- PMID: 20519694
- PMCID: PMC2957158
- DOI: 10.1095/biolreprod.110.084418
Cumulus-oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro
Abstract
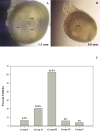
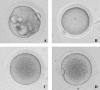
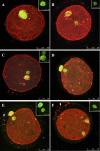
The stage at which follicle-enclosed cumulus-oocyte complexes achieve developmental competence in primates is unknown. Therefore, studies were designed to characterize the ability of oocytes in small antral follicles present during the menstrual cycle to spontaneously resume meiosis, fertilize, and support early embryo development. Ovaries were removed from adult rhesus monkeys (n = 12) during the early follicular phase (Days 3-4) of spontaneous cycles. Small antral follicles were divided into five groups according to their diameter; group I: <0.5 mm; group II: 0.5-0.99 mm; group III: 1.0-1.49 mm; group IV: 1.5-1.99 mm; and group V: 2.0-2.5 mm. The cumulus-oocyte complex from healthy small antral follicles (devoid of dark oocytes or granulosa cells) were extracted (n = 199) and cultured for 48 h under different conditions: in TALP (tyrode, albumin, lactate, pyruvate) medium alone, SAGE medium alone, or plus gonadotropins. At 48 h, oocyte meiotic status and diameter were measured after treatment of cumulus-oocyte complexes with hyaluronidase. Cumulus-oocyte complexes derived from follicles of 0.5- to 2-mm diameter contain oocytes that typically reinitiate meiosis in the absence or presence of gonadotropins and fertilize via in vitro fertilization or intracytoplasmic sperm injection. Moreover, the inseminated oocytes can reach the morula stage but arrest. Thus, the ability of these oocytes to complete maturation, as monitored from subsequent embryonic development after fertilization, is suboptimal. Further studies on primate IVM of oocytes from SAFs are warranted in order for them to be considered as an additional, novel source of gametes for fertility preservation in cancer patients.
Figures

References
-
- McVie JG.Cancer treatment: the last 25 years. Cancer Treat Rev 1999; 25: 323–331. - PubMed
-
- Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlander N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, et al. SEER cancer statistics review, 1975–2005. National Cancer Institute, Bethesda, MD,http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER Web site,2008.
-
- Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN.Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab 2003; 88: 5307–5314. - PubMed
-
- Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Kort HI, Vajta G.The efficacy and safety of human oocyte vitrification. Semin Reprod Med 2009; 27: 450–455. - PubMed
Publication types
MeSH terms
Grants and funding
- RL1 HD058294/HD/NICHD NIH HHS/United States
- P51 RR000163/RR/NCRR NIH HHS/United States
- R01-HD058294/HD/NICHD NIH HHS/United States
- RL1HD058295/HD/NICHD NIH HHS/United States
- RR00163/RR/NCRR NIH HHS/United States
- PL1 EB008542/EB/NIBIB NIH HHS/United States
- K01 RR000163/RR/NCRR NIH HHS/United States
- RL1 HD058295/HD/NICHD NIH HHS/United States
- U54 HD18185/HD/NICHD NIH HHS/United States
- U54 HD055744/HD/NICHD NIH HHS/United States
- UL1DE019587/DE/NIDCR NIH HHS/United States
- U54 HD018185/HD/NICHD NIH HHS/United States
- PL1-EB008542/EB/NIBIB NIH HHS/United States
- U54 HD55744/HD/NICHD NIH HHS/United States
- T32-HD007068-3/HD/NICHD NIH HHS/United States
- UL1 DE019587/DE/NIDCR NIH HHS/United States
- UL1 RR024926/RR/NCRR NIH HHS/United States
- T32 HD007068/HD/NICHD NIH HHS/United States
- 1 UL1 RR024926/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources

