Cardiotoxicity of the anticancer therapeutic agent bortezomib
- PMID: 20519734
- PMCID: PMC2877829
- DOI: 10.2353/ajpath.2010.090690
Cardiotoxicity of the anticancer therapeutic agent bortezomib
Abstract
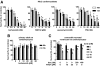
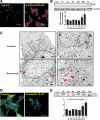
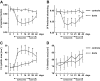
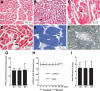
Recent case reports provided alarming signals that treatment with bortezomib might be associated with cardiac events. In all reported cases, patients experiencing cardiac problems were previously or concomitantly treated with other chemotherapeutics including cardiotoxic anthracyclines. Therefore, it is difficult to distinguish which components of the therapeutic regimens contribute to cardiotoxicity. Here, we addressed the influence of bortezomib on cardiac function in rats that were not treated with other drugs. Rats were treated with bortezomib at a dose of 0.2 mg/kg thrice weekly. Echocardiography, histopathology, and electron microscopy were used to evaluate cardiac function and structural changes. Respiration of the rat heart mitochondria was measured polarographically. Cell culture experiments were used to determine the influence of bortezomib on cardiomyocyte survival, contractility, Ca(2+) fluxes, induction of endoplasmic reticulum stress, and autophagy. Our findings indicate that bortezomib treatment leads to left ventricular contractile dysfunction manifested by a significant drop in left ventricle ejection fraction. Dramatic ultrastructural abnormalities of cardiomyocytes, especially within mitochondria, were accompanied by decreased ATP synthesis and decreased cardiomyocyte contractility. Monitoring of cardiac function in bortezomib-treated patients should be implemented to evaluate how frequently cardiotoxicity develops especially in patients with pre-existing cardiac conditions, as well as when using additional cardiotoxic drugs.
Figures

References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. - PubMed
-
- Mego M, Reckova M, Obertova J, Sycova-Mila Z, Brozmanova K, Mardiak J. Increased cardiotoxicity of sorafenib in sunitinib-pretreated patients with metastatic renal cell carcinoma. Ann Oncol. 2007;18:1906–1907. - PubMed
-
- Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. - PubMed
-
- Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, Canales C, Daud A, Spriggs DR. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

