Circadian clock proteins control adaptation to novel environment and memory formation
- PMID: 20519775
- PMCID: PMC2898019
- DOI: 10.18632/aging.100142
Circadian clock proteins control adaptation to novel environment and memory formation
Abstract
Deficiency of the transcription factor BMAL1, a core component of the circadian clock, results in an accelerated aging phenotype in mice. The circadian clock regulates many physiological processes and was recently implicated in control of brain-based activities, such as memory formation and the regulation of emotions. Aging is accompanied by the decline in brain physiology, particularly decline in the response and adaptation to novelty. We investigated the role of the circadian clock in exploratory behavior and habituation to novelty using the open field paradigm. We found that mice with a deficiency of the circadian transcription factor BMAL1 display hyperactivity in novel environments and impaired intra- and intersession habituation, indicative of defects in short- and long-term memory formation. In contrast, mice double-deficient for the circadian proteins CRY1 and CRY2 (repressors of the BMAL1-mediated transcription) demonstrate reduced activity and accelerated habituation when compared to wild type mice. Mice with mutation in theClock gene (encoding the BMAL1 transcription partner) show normal locomotion, but increased rearing activity and impaired intersession habituation. BMAL1 is highly expressed in the neurons of the hippocampus - a brain region associated with spatial memory formation; BMAL1 deficiency disrupts circadian oscillation in gene expression and reactive oxygen species homeostasis in the brain, which may be among the possible mechanisms involved. Thus, we suggest that the BMAL1:CLOCK activity is critical for the proper exploratory and habituation behavior, and that the circadian clock prepares organism for a new round of everyday activities through optimization of behavioral learning.
Conflict of interest statement
The authors of this manuscript have no conflict of interests to declare.
Figures
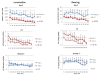




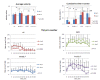
Comment in
-
The aging clock: to 'BMAL'icious toward learning and memory.Aging (Albany NY). 2010 May;2(5):251-4. doi: 10.18632/aging.100144. Aging (Albany NY). 2010. PMID: 20519776 Free PMC article. No abstract available.
-
Ageing or NOT, clock genes are important for memory processes: an interesting hypothesis raising many questions.Aging (Albany NY). 2010 May;2(5):259-60. doi: 10.18632/aging.100148. Aging (Albany NY). 2010. PMID: 20519780 Free PMC article. No abstract available.
-
Aging brains and waning clocks on the process of habituation.Aging (Albany NY). 2010 Jun;2(6):320-1. doi: 10.18632/aging.100154. Aging (Albany NY). 2010. PMID: 20603522 Free PMC article. No abstract available.
Similar articles
-
Temporal dynamics of mouse hippocampal clock gene expression support memory processing.Hippocampus. 2010 Mar;20(3):377-88. doi: 10.1002/hipo.20637. Hippocampus. 2010. PMID: 19437502
-
The circadian clock regulates rhythmic erythropoietin expression in the murine kidney.Kidney Int. 2021 Nov;100(5):1071-1080. doi: 10.1016/j.kint.2021.07.012. Epub 2021 Jul 30. Kidney Int. 2021. PMID: 34332958
-
The Arg-293 of Cryptochrome1 is responsible for the allosteric regulation of CLOCK-CRY1 binding in circadian rhythm.J Biol Chem. 2020 Dec 11;295(50):17187-17199. doi: 10.1074/jbc.RA120.014333. Epub 2020 Oct 7. J Biol Chem. 2020. PMID: 33028638 Free PMC article.
-
The Circadian Clock, Nutritional Signals and Reproduction: A Close Relationship.Int J Mol Sci. 2023 Jan 12;24(2):1545. doi: 10.3390/ijms24021545. Int J Mol Sci. 2023. PMID: 36675058 Free PMC article. Review.
-
[Molecular mechanisms of circadian clock functioning].Ukr Biokhim Zh (1999). 2011 May-Jun;83(3):5-24. Ukr Biokhim Zh (1999). 2011. PMID: 21888051 Review. Ukrainian.
Cited by
-
Circadian regulation of membrane physiology in neural oscillators throughout the brain.Eur J Neurosci. 2020 Jan;51(1):109-138. doi: 10.1111/ejn.14343. Epub 2019 Jan 29. Eur J Neurosci. 2020. PMID: 30633846 Free PMC article. Review.
-
Modulation of learning and memory by the genetic disruption of circadian oscillator populations.Physiol Behav. 2018 Oct 1;194:387-393. doi: 10.1016/j.physbeh.2018.06.035. Epub 2018 Jun 23. Physiol Behav. 2018. PMID: 29944860 Free PMC article.
-
Glial Cells in the Genesis and Regulation of Circadian Rhythms.Front Physiol. 2018 Feb 12;9:88. doi: 10.3389/fphys.2018.00088. eCollection 2018. Front Physiol. 2018. PMID: 29483880 Free PMC article. Review.
-
Aging brains and waning clocks on the process of habituation.Aging (Albany NY). 2010 Jun;2(6):320-1. doi: 10.18632/aging.100154. Aging (Albany NY). 2010. PMID: 20603522 Free PMC article. No abstract available.
-
Adult Neurogenesis under Control of the Circadian System.Cells. 2022 Feb 22;11(5):764. doi: 10.3390/cells11050764. Cells. 2022. PMID: 35269386 Free PMC article. Review.
References
-
- Cerbone A, Sadile AG. Behavioral habituation to spatial novelty: interference and noninterference studies. Neurosci Biobehav Rev. 1994;18:497–518. - PubMed
-
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev, 2006;30:1045–1064. - PubMed
-
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson RF. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92:135–138. - PMC - PubMed
-
- Kondratov RV, Gorbacheva VY, Antoch MP. The role of mammalian circadian proteins in normal physiology and genotoxic stress responses. Curr Top Dev Biol. 2007;78:173–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

