SPLUNC1 expression reduces surface levels of the epithelial sodium channel (ENaC) in Xenopus laevis oocytes
- PMID: 20519934
- PMCID: PMC2975823
- DOI: 10.4161/chan.4.4.12255
SPLUNC1 expression reduces surface levels of the epithelial sodium channel (ENaC) in Xenopus laevis oocytes
Abstract
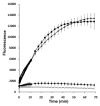
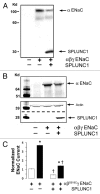
Throughout the body, the epithelial Na(+) channel (ENaC) plays a critical role in salt and liquid homeostasis. In cystic fibrosis airways, for instance, improper regulation of ENaC results in hyperabsorption of sodium that causes dehydration of airway surface liquid. This dysregulation then contributes to mucus stasis and chronic lung infections. ENaC is known to undergo proteolytic cleavage, which is required for its ability to conduct Na(+) ions. We have previously shown that the short, palate lung and nasal epithelial clone (SPLUNC1) binds to and inhibits ENaC in both airway epithelia and in Xenopus laevis oocytes. In this study, we found that SPLUNC1 was more potent at inhibiting ENaC than either SPLUNC2 or long PLUNC1 (LPLUNC1), two other PLUNC family proteins that are also expressed in airway epithelia. Furthermore, we were able to shed light on the potential mechanism of SPLUNC1's inhibition of ENaC. While SPLUNC1 did not inhibit proteolytic activity of trypsin, it significantly reduced ENaC currents by reducing the number of ENaCs in the plasma membrane. A better understanding of ENaC's regulation by endogenous inhibitors may aid in the development of novel therapies designed to inhibit hyperactive ENaC in cystic fibrosis epithelia.
Figures
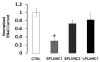
Comment on
-
SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage.Proc Natl Acad Sci U S A. 2009 Jul 7;106(27):11412-7. doi: 10.1073/pnas.0903609106. Epub 2009 Jun 18. Proc Natl Acad Sci U S A. 2009. PMID: 19541605 Free PMC article.
Similar articles
-
SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage.Proc Natl Acad Sci U S A. 2009 Jul 7;106(27):11412-7. doi: 10.1073/pnas.0903609106. Epub 2009 Jun 18. Proc Natl Acad Sci U S A. 2009. PMID: 19541605 Free PMC article.
-
SPLUNC1 is an allosteric modulator of the epithelial sodium channel.FASEB J. 2018 May;32(5):2478-2491. doi: 10.1096/fj.201701126R. Epub 2018 Jan 8. FASEB J. 2018. PMID: 29295861 Free PMC article.
-
SPLUNC1 degradation by the cystic fibrosis mucosal environment drives airway surface liquid dehydration.Eur Respir J. 2018 Oct 4;52(4):1800668. doi: 10.1183/13993003.00668-2018. Print 2018 Oct. Eur Respir J. 2018. PMID: 30190268 Free PMC article.
-
Distribution of human PLUNC/BPI fold-containing (BPIF) proteins.Biochem Soc Trans. 2011 Aug;39(4):1023-7. doi: 10.1042/BST0391023. Biochem Soc Trans. 2011. PMID: 21787341 Review.
-
Mammalian short palate lung and nasal epithelial clone 1 (SPLUNC1) in pH-dependent airway hydration.Int J Biochem Cell Biol. 2014 Jul;52:130-5. doi: 10.1016/j.biocel.2014.03.002. Epub 2014 Mar 13. Int J Biochem Cell Biol. 2014. PMID: 24631954 Free PMC article. Review.
Cited by
-
Enhanced biofilm prevention activity of a SPLUNC1-derived antimicrobial peptide against Staphylococcus aureus.PLoS One. 2018 Sep 14;13(9):e0203621. doi: 10.1371/journal.pone.0203621. eCollection 2018. PLoS One. 2018. PMID: 30216370 Free PMC article.
-
Inhaled Biologicals for the Treatment of Cystic Fibrosis.Recent Pat Inflamm Allergy Drug Discov. 2019;13(1):19-26. doi: 10.2174/1872213X12666181012101444. Recent Pat Inflamm Allergy Drug Discov. 2019. PMID: 30318010 Free PMC article. Review.
-
Identification of SPLUNC1's ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airways.FASEB J. 2012 Oct;26(10):4348-59. doi: 10.1096/fj.12-207431. Epub 2012 Jul 13. FASEB J. 2012. Retraction in: FASEB J. 2013 May;27(5):2081. doi: 10.1096/fj.12-207431RET. PMID: 22798424 Free PMC article. Retracted.
-
Evaluation of a SPLUNC1-derived peptide for the treatment of cystic fibrosis lung disease.Am J Physiol Lung Cell Mol Physiol. 2018 Jan 1;314(1):L192-L205. doi: 10.1152/ajplung.00546.2016. Epub 2017 Oct 5. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 28982737 Free PMC article.
-
BPIFB1 (LPLUNC1) is upregulated in cystic fibrosis lung disease.Histochem Cell Biol. 2012 Nov;138(5):749-58. doi: 10.1007/s00418-012-0990-8. Epub 2012 Jul 6. Histochem Cell Biol. 2012. PMID: 22767025 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
