Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors
- PMID: 20519948
- PMCID: PMC3319752
- DOI: 10.4161/cc.9.12.11988
Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors
Abstract
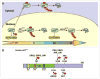
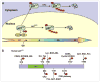
The expression levels of the p21(Cip1) family CDK inhibitors (CKIs), p21(Cip1), p27(Kip1) and p57(Kip2), play a pivotal role in the precise regulation of cyclin-dependent kinase (CDK) activity, which is instrumental to proper cell cycle progression. The stabilities of p21(Cip1), p27(Kip1) and p57(Kip2) are all tightly and differentially regulated by ubiquitylation and proteasome-mediated degradation during various stages of the cell cycle, either in steady state or in response to extracellular stimuli, which often elicit site-specific phosphorylation of CKIs triggering their degradation.
Figures

References
-
- Jackson PK, Eldridge AG, Freed E, Furstenthal L, Hsu JY, Kaiser BK, et al. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 2000;10:429–39. - PubMed
-
- Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI. U box proteins as a new family of ubiquitin-protein ligases. J Biol Chem. 2001;276:33111–20. - PubMed
-
- Boname JM, Stevenson PG. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity. 2001;15:627–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
