Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α
- PMID: 20519961
- PMCID: PMC3180089
- DOI: 10.4161/mabs.12304
Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α
Abstract
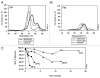
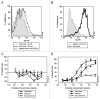
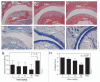
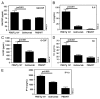
We prepared and characterized golimumab (CNTO148), a human IgG1 tumor necrosis factor alpha (TNFα) antagonist monoclonal antibody chosen for clinical development based on its molecular properties. Golimumab was compared with infliximab, adalimumab and etanercept for affinity and in vitro TNFα neutralization. The affinity of golimumab for soluble human TNFα, as determined by surface plasmon resonance, was similar to that of etanercept (18 pM versus 11 pM), greater than that of infliximab (44 pM) and significantly greater than that of adalimumab (127 pM, p=0.018). The concentration of golimumab necessary to neutralize TNFα-induced E-selectin expression on human endothelial cells by 50% was significantly less than those for infliximab (3.2 fold; p=0.017) and adalimumab (3.3-fold; p=0.008) and comparable to that for etanercept. The conformational stability of golimumab was greater than that of infliximab (primary melting temperature [Tm] 74.8 °C vs. 69.5 °C) as assessed by differential scanning calorimetry. In addition, golimumab showed minimal aggregation over the intended shelf life when formulated as a high concentration liquid product (100 mg/mL) for subcutaneous administration. In vivo, golimumab at doses of 1 and 10 mg/kg significantly delayed disease progression in a mouse model of human TNFα-induced arthritis when compared with untreated mice, while infliximab was effective only at 10 mg/kg. Golimumab also significantly reduced histological scores for arthritis severity and cartilage damage, as well as serum levels of pro-inflammatory cytokines and chemokines associated with arthritis. Thus, we have demonstrated that golimumab is a highly stable human monoclonal antibody with high affinity and capacity to neutralize human TNFα in vitro and in vivo.
Figures


References
-
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. - PubMed
-
- Feldmann M, Brennan FM, Williams RO, Woody JN, Maini RN. The transfer of a laboratory based hypothesis to a clinically useful therapy: the development of anti-TNF therapy of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2004;18:59–80. - PubMed
-
- Ackermann C, Kavanaugh A. Tumor necrosis factor as a therapeutic target of rheumatologic disease. Expert Opin Ther Targets. 2007;11:1369–1384. - PubMed
-
- Chang JT, Lichtenstein GR. Drug insight: antagonists of tumor-necrosis factor-alpha in the treatment of inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:220–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
