Phase I trial of a humanized, Fc receptor nonbinding anti-CD3 antibody, hu12F6mu in patients receiving renal allografts
- PMID: 20519962
- PMCID: PMC3180091
- DOI: 10.4161/mabs.12305
Phase I trial of a humanized, Fc receptor nonbinding anti-CD3 antibody, hu12F6mu in patients receiving renal allografts
Abstract
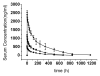
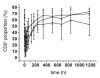
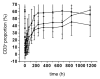
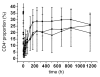
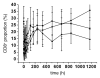
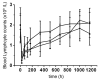
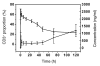
Hu12F6mu is an Fc-mutated, humanized anti-CD3 antibody developed in our lab. The aim of this study was to assess single dose escalation pharmacokinetics (PK) and safety profile of hu12F6mu and to measure the effects of the antibody on levels of circulating T cells over time. Twenty-seven patients receiving renal allografts were randomized to receive hu12F6mu intravenously at a single-dose of 2.5, 5 or 10 mg. The concentration-time data obtained by a validated ELISA method were subjected to non-compartmental PK analysis by DAS 2.1 software. Subgroups of CD2(+), CD3(+), CD4(+) and CD8(+) lymphocytes were monitored periodically by flow cytometry. Our results showed that hu12F6mu exhibited linear PK over the dose range of 2.5 to 10 mg. A significant decline in the proportion of T cells was observed immediately after the infusion, followed by a progressive increase occurring over the ensuing days of therapy. A significant negative correlation was observed between serum concentration of hu12F6mu and CD3(+) cell proportion. Intravenous infusion of hu12F6mu was well-tolerated in patients receiving renal allografts. These results suggest that hu12F6mu may have potential as a therapeutic agent, although further studies are needed.
Figures
References
-
- Cosimi AB. Clinical development of Orthoclone OKT3. Transplant Proc. 1987;19:7–16. - PubMed
-
- Colonna JO, Goldstein LI, Brems JJ, Vargas JH, Brill JE, Berquist WJ, et al. A prospective study on the use of monoclonal anti-T3-cell antibody (OKT3) to treat steroid-resistant liver transplant rejection. Arch Surg. 1987;112:1120–1123. - PubMed
-
- Cosimi AB, Burton RC, Colvin RB, Goldstein G, Delmonico FL, LaQuaglia MP, et al. Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation. 1981;32:535–539. - PubMed
-
- Cosimi AB, Cho SI, Delmonico FL, Kaplan MM, Rohrer RJ, Jenkins RL. A randomized clinical trial comparing OKT3 and steroids for treatment of hepatic allograft rejection. Transplantation Proc. 1987;19:2431–2433. - PubMed
-
- Delmonico FL, Auchincloss H, Yang H, Russell PS, Cosimi AB. Indications and outcome of OKT3 therapy after liver, pancreas and renal transplantation. Transplant Proc. 1988;20:249–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
