All things must pass: contrasts and commonalities in eukaryotic and bacterial mRNA decay
- PMID: 20520623
- PMCID: PMC3145457
- DOI: 10.1038/nrm2917
All things must pass: contrasts and commonalities in eukaryotic and bacterial mRNA decay
Abstract
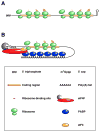
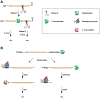
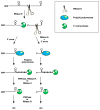
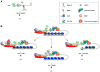
Despite its universal importance for controlling gene expression, mRNA degradation was initially thought to occur by disparate mechanisms in eukaryotes and bacteria. This conclusion was based on differences in the structures used by these organisms to protect mRNA termini and in the RNases and modifying enzymes originally implicated in mRNA decay. Subsequent discoveries have identified several striking parallels between the cellular factors and molecular events that govern mRNA degradation in these two kingdoms of life. Nevertheless, some key distinctions remain, the most fundamental of which may be related to the different mechanisms by which eukaryotes and bacteria control translation initiation.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

