Optimisation of bioluminescent reporters for use with mycobacteria
- PMID: 20520722
- PMCID: PMC2875389
- DOI: 10.1371/journal.pone.0010777
Optimisation of bioluminescent reporters for use with mycobacteria
Abstract
Background: Mycobacterium tuberculosis, the causative agent of tuberculosis, still represents a major public health threat in many countries. Bioluminescence, the production of light by luciferase-catalyzed reactions, is a versatile reporter technology with multiple applications both in vitro and in vivo. In vivo bioluminescence imaging (BLI) represents one of its most outstanding uses by allowing the non-invasive localization of luciferase-expressing cells within a live animal. Despite the extensive use of luminescent reporters in mycobacteria, the resultant luminescent strains have not been fully applied to BLI.
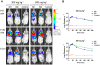
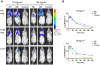
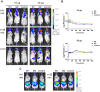
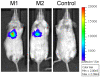
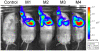
Methodology/principal findings: One of the main obstacles to the use of bioluminescence for in vivo imaging is the achievement of reporter protein expression levels high enough to obtain a signal that can be detected externally. Therefore, as a first step in the application of this technology to the study of mycobacterial infection in vivo, we have optimised the use of firefly, Gaussia and bacterial luciferases in mycobacteria using a combination of vectors, promoters, and codon-optimised genes. We report for the first time the functional expression of the whole bacterial lux operon in Mycobacterium tuberculosis and M. smegmatis thus allowing the development of auto-luminescent mycobacteria. We demonstrate that the Gaussia luciferase is secreted from bacterial cells and that this secretion does not require a signal sequence. Finally we prove that the signal produced by recombinant mycobacteria expressing either the firefly or bacterial luciferases can be non-invasively detected in the lungs of infected mice by bioluminescence imaging.
Conclusions/significance: While much work remains to be done, the finding that both firefly and bacterial luciferases can be detected non-invasively in live mice is an important first step to using these reporters to study the pathogenesis of M. tuberculosis and other mycobacterial species in vivo. Furthermore, the development of auto-luminescent mycobacteria has enormous ramifications for high throughput mycobacterial drug screening assays which are currently carried out either in a destructive manner using LuxAB or the firefly luciferase.
Conflict of interest statement
Figures





References
-
- WHO. Global tuberculosis control 2009. 2009. 303. Epidemiology, strategy, financing: WHO Press.
-
- Hutchens M, Luker GD. Applications of bioluminescence imaging to the study of infectious diseases. Cell Microbiol. 2007;9:2315–2322. - PubMed
-
- Steinhuber A, Landmann R, Goerke C, Wolz C, Fluckiger U. Bioluminescence imaging to study the promoter activity of hla of Staphylococcus aureus in vitro and in vivo. Int J Med Microbiol. 2008;298:599–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

