Morphological and molecular characterizations of psychrophilic fungus Geomyces destructans from New York bats with White Nose Syndrome (WNS)
- PMID: 20520731
- PMCID: PMC2875398
- DOI: 10.1371/journal.pone.0010783
Morphological and molecular characterizations of psychrophilic fungus Geomyces destructans from New York bats with White Nose Syndrome (WNS)
Abstract
Background: Massive die-offs of little brown bats (Myotis lucifugus) have been occurring since 2006 in hibernation sites around Albany, New York, and this problem has spread to other States in the Northeastern United States. White cottony fungal growth is seen on the snouts of affected animals, a prominent sign of White Nose Syndrome (WNS). A previous report described the involvement of the fungus Geomyces destructans in WNS, but an identical fungus was recently isolated in France from a bat that was evidently healthy. The fungus has been recovered sparsely despite plentiful availability of afflicted animals.
Methodology/principal findings: We have investigated 100 bat and environmental samples from eight affected sites in 2008. Our findings provide strong evidence for an etiologic role of G. destructans in bat WNS. (i) Direct smears from bat snouts, Periodic Acid Schiff-stained tissue sections from infected tissues, and scanning electron micrographs of bat tissues all showed fungal structures similar to those of G. destructans. (ii) G. destructans DNA was directly amplified from infected bat tissues, (iii) Isolations of G. destructans in cultures from infected bat tissues showed 100% DNA match with the fungus present in positive tissue samples. (iv) RAPD patterns for all G. destructans cultures isolated from two sites were indistinguishable. (v) The fungal isolates showed psychrophilic growth. (vi) We identified in vitro proteolytic activities suggestive of known fungal pathogenic traits in G. destructans.
Conclusions/significance: Further studies are needed to understand whether G. destructans WNS is a symptom or a trigger for bat mass mortality. The availability of well-characterized G. destructans strains should promote an understanding of bat-fungus relationships, and should aid in the screening of biological and chemical control agents.
Conflict of interest statement
Figures
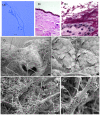




 and France
and France  . (B) Phylogenetic tree constructed by parsimony analysis of 28S ribosomal sequences. The evolutionary history of representative isolates of G. destructans from this study and additional related fungi sequenced in our laboratory, were inferred using the Maximum Parsimony method and bootstrap consensus tree from 1000 replicates conducted in MEGA 4.1. After elimination of gaps and missing data, the dataset contained 537 positions of which 88 were parsimony informative. The consensus phylogenetic tree shown was inferred from 94 most parsimonious trees.
. (B) Phylogenetic tree constructed by parsimony analysis of 28S ribosomal sequences. The evolutionary history of representative isolates of G. destructans from this study and additional related fungi sequenced in our laboratory, were inferred using the Maximum Parsimony method and bootstrap consensus tree from 1000 replicates conducted in MEGA 4.1. After elimination of gaps and missing data, the dataset contained 537 positions of which 88 were parsimony informative. The consensus phylogenetic tree shown was inferred from 94 most parsimonious trees.

Similar articles
-
DNA-based detection of the fungal pathogen Geomyces destructans in soils from bat hibernacula.Mycologia. 2011 Mar-Apr;103(2):241-6. doi: 10.3852/10-262. Epub 2010 Oct 7. Mycologia. 2011. PMID: 20952799
-
Experimental infection of bats with Geomyces destructans causes white-nose syndrome.Nature. 2011 Oct 26;480(7377):376-8. doi: 10.1038/nature10590. Nature. 2011. PMID: 22031324
-
Distribution and environmental persistence of the causative agent of white-nose syndrome, Geomyces destructans, in bat hibernacula of the eastern United States.Appl Environ Microbiol. 2013 Feb;79(4):1293-301. doi: 10.1128/AEM.02939-12. Epub 2012 Dec 14. Appl Environ Microbiol. 2013. PMID: 23241985 Free PMC article.
-
Investigating and managing the rapid emergence of white-nose syndrome, a novel, fatal, infectious disease of hibernating bats.Conserv Biol. 2011 Apr;25(2):223-31. doi: 10.1111/j.1523-1739.2010.01638.x. Epub 2011 Feb 1. Conserv Biol. 2011. PMID: 21284732 Review.
-
COULD WHITE-NOSE SYNDROME MANIFEST DIFFERENTLY IN MYOTIS LUCIFUGUS IN WESTERN VERSUS EASTERN REGIONS OF NORTH AMERICA? A REVIEW OF FACTORS.J Wildl Dis. 2023 Jul 1;59(3):381-397. doi: 10.7589/JWD-D-22-00050. J Wildl Dis. 2023. PMID: 37270186 Review.
Cited by
-
The Effects of Cutaneous Fatty Acids on the Growth of Pseudogymnoascus destructans, the Etiological Agent of White-Nose Syndrome (WNS).PLoS One. 2016 Apr 12;11(4):e0153535. doi: 10.1371/journal.pone.0153535. eCollection 2016. PLoS One. 2016. PMID: 27070905 Free PMC article.
-
Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection.Sci Rep. 2016 Sep 13;6:33200. doi: 10.1038/srep33200. Sci Rep. 2016. PMID: 27620349 Free PMC article.
-
Lipolytic Activity and the Utilization of Fatty Acids Associated with Bat Sebum by Pseudogymnoascus destructans.Mycopathologia. 2019 Oct;184(5):625-636. doi: 10.1007/s11046-019-00381-4. Epub 2019 Sep 16. Mycopathologia. 2019. PMID: 31529298
-
Novel Trichoderma polysporum Strain for the Biocontrol of Pseudogymnoascus destructans, the Fungal Etiologic Agent of Bat White Nose Syndrome.PLoS One. 2015 Oct 28;10(10):e0141316. doi: 10.1371/journal.pone.0141316. eCollection 2015. PLoS One. 2015. PMID: 26509269 Free PMC article.
-
Isolation and identification of an extracellular subtilisin-like serine protease secreted by the bat pathogen Pseudogymnoascus destructans.PLoS One. 2015 Mar 18;10(3):e0120508. doi: 10.1371/journal.pone.0120508. eCollection 2015. PLoS One. 2015. PMID: 25785714 Free PMC article.
References
-
- Kunz TH, Fenton MB. Chicago, IL: University of Chicago Press; 2003. Bat Ecology.779
-
- Williams-Guillen K, Perfecto I, Vandermeer J. Bats limit insects in a neotropical agroforestry system. Science. 2008;320:70. - PubMed
-
- Jülg B, Elias J, Zahn A, Köppen S, Becker-Gaab C, et al. Bat-associated histoplasmosis can be transmitted at entrances of bat caves and not only inside the caves. J Travel Med. 2008;15:133–136. - PubMed
-
- Chaturvedi VP, Randhawa HS, Khan ZU, Singh N, Kini S. Prevalence of Basidiobolus ranarum Eidam in the intestinal tract of an insectivorous bat, Rhinopoma hardwickei hardwickei Gray, in Delhi. Sabouraudia. 1984;22:185–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

