Cell-free expression of protein kinase a for rapid activity assays
- PMID: 20520741
- PMCID: PMC2879223
- DOI: 10.4137/aci.s4732
Cell-free expression of protein kinase a for rapid activity assays
Abstract
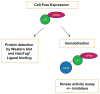
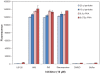
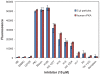
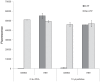
Functional protein analysis often calls for lengthy, laborious in vivo protein expression and purification, and can be complicated by the lack of stability of the purified protein. In this study, we demonstrate the feasibility of a simplified procedure for functional protein analysis on magnetic particles using cell-free protein synthesis of the catalytic subunit of human cAMP-dependent protein kinase as a HaloTag((R)) fusion protein. The cell-free protein synthesis systems provide quick access to the protein of interest, while the HaloTag technology provides efficient, covalent protein immobilization of the fusion protein, eliminating the need for further protein purification and minimizing storage-related stability issues. The immobilized cPKA fusion protein is assayed directly on magnetic beads and can be used in inhibitor analyses. The combination of rapid protein synthesis and capture technologies can greatly facilitate the process of protein expression and activity screening, and therefore, can become a valuable tool for functional proteomics studies.
Keywords: HaloTag; PKA; cell-free expression; in vitro translation; kinase; magnetic particles; protein immobilization.
Figures



Similar articles
-
HaloTag technology: a versatile platform for biomedical applications.Bioconjug Chem. 2015 Jun 17;26(6):975-86. doi: 10.1021/acs.bioconjchem.5b00191. Epub 2015 May 22. Bioconjug Chem. 2015. PMID: 25974629 Free PMC article. Review.
-
Expression, one-step purification, and immobilization of HaloTag(TM) fusion proteins on chloroalkane-functionalized magnetic beads.Appl Biochem Biotechnol. 2010 Nov;162(7):2098-110. doi: 10.1007/s12010-010-8985-1. Epub 2010 May 15. Appl Biochem Biotechnol. 2010. PMID: 20473582
-
Purification of Recombinant Proteins from Cultured Mammalian Cells by HaloTag Technology.Curr Protoc Mol Biol. 2015 Apr 1;110:10.31.1-10.31.15. doi: 10.1002/0471142727.mb1031s110. Curr Protoc Mol Biol. 2015. PMID: 25827085
-
HaloTag-based purification of functional human kinases from mammalian cells.Protein Expr Purif. 2011 Apr;76(2):154-64. doi: 10.1016/j.pep.2010.11.014. Epub 2010 Dec 1. Protein Expr Purif. 2011. PMID: 21129486
-
[The cell-free protein synthesis-based protein microarray technology].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010 Dec;27(6):1397-400, 1409. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010. PMID: 21375003 Review. Chinese.
Cited by
-
HaloTag technology: a versatile platform for biomedical applications.Bioconjug Chem. 2015 Jun 17;26(6):975-86. doi: 10.1021/acs.bioconjchem.5b00191. Epub 2015 May 22. Bioconjug Chem. 2015. PMID: 25974629 Free PMC article. Review.
-
Tagging of functional ribosomes in living cells by HaloTag® technology.In Vitro Cell Dev Biol Anim. 2011 Feb;47(2):132-8. doi: 10.1007/s11626-010-9370-7. Epub 2010 Nov 17. In Vitro Cell Dev Biol Anim. 2011. PMID: 21082278
-
A dynamic study of protein secretion and aggregation in the secretory pathway.PLoS One. 2014 Oct 3;9(10):e108496. doi: 10.1371/journal.pone.0108496. eCollection 2014. PLoS One. 2014. PMID: 25279560 Free PMC article.
-
Single-Molecule Fluorescence Detection of the Epidermal Growth Factor Receptor in Membrane Discs.Biochemistry. 2019 Jan 29;58(4):286-294. doi: 10.1021/acs.biochem.8b00089. Epub 2018 Apr 6. Biochemistry. 2019. PMID: 29553754 Free PMC article.
-
In Vitro Kinase Assay with Recombinant SnRK2s: An Example for Assaying Stress-Responsive Kinases in Plants.Methods Mol Biol. 2024;2832:163-170. doi: 10.1007/978-1-0716-3973-3_11. Methods Mol Biol. 2024. PMID: 38869794
References
-
- Arduengo M, Schenborn E, Hurst R. The Role of Cell-Free Rabbit Reticulocyte Expression Systems in Functional Proteomics. In: Kudlicki W, Katzen W, Bennett R, editors. Cell-Free Expression. Vol. 2007. Austin, TX: Landes Bioscience; 2007. pp. 1–18.
-
- Litterer L. Expressing Mammalian Proteins Using Insect and Rabbit Cell-Free Lysates. Promega Notes. 2009;101:19–21.
-
- Hurst R, Creswell D, Slater MR, Schenborn E. TNT® SP6 High-Yield Protein Expression System: More protein from a coupled transcription/translation system. Promega Notes. 2006;93:15–8.
-
- Zhao KQ, Hurst R, Slater MR, Bulleit RF. Functional protein expression from a DNA based wheat germ cell-free system. J Struc Funct Genomics. 2007;8:199–208. - PubMed
-
- Leippe D, Creswell D, Hartnett J, Schenborn E. Cell-Free Protein Expression with the TNT® T7 Insect Cell Extract Protein Expression System. Promega Notes. 2008;100:11–2.
LinkOut - more resources
Full Text Sources

