Responsiveness to PI3K and MEK inhibitors in breast cancer. Use of a 3D culture system to study pathways related to hormone independence in mice
- PMID: 20520761
- PMCID: PMC2877092
- DOI: 10.1371/journal.pone.0010786
Responsiveness to PI3K and MEK inhibitors in breast cancer. Use of a 3D culture system to study pathways related to hormone independence in mice
Abstract
Background: A significant proportion of breast cancer patients face failure of endocrine therapy due to the acquisition of endocrine resistance. We have explored mechanisms involved in such disease progression by using a mouse breast cancer model that is induced by medroxyprogesterone acetate (MPA). These tumors transit through different stages of hormone sensitivity. However, when cells from tumor variants were seeded on plastic, all were stimulated by progestins and inhibited by antiprogestins such as RU486. Furthermore, cells from a RU486-resistant tumor variant recovered antiprogestin sensitivity.
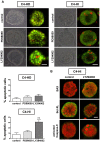
Hypothesis: A three-dimensional (3D) culture system, by maintaining differential cellular organization that is typical of each tumor variant, may allow for the maintenance of particular hormone responses and thus be appropriate for the study of the effects of specific inhibitors of signaling pathways associated with disease progression.
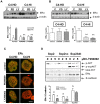
Method: We compared the behavior of tumors growing in vivo and cancer cells ex vivo (in 3D Matrigel). In this system, we evaluated the effects of kinase inhibitors and hormone antagonists on tumor growth.
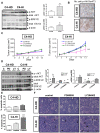
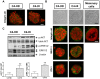
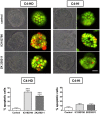
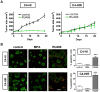
Principal findings: LY294002, a PI3K/AKT pathway inhibitor, decreased both tumor growth in vivo and cell survival in Matrigel in MPA-independent tumors with higher AKT activity. Induction of cell death by anti-hormones such as ICI182780 and ZK230211 was more effective in MPA-dependent tumors with lower AKT activity. Inhibition of MEK with PD98059 did not affect tumor growth in any tested variant. Finally, while Matrigel reproduced differential responsiveness of MPA-dependent and -independent breast cancer cells, it was not sufficient to preserve antiprogestin resistance of RU486-resistant tumors.
Conclusion: We demonstrated that the PI3K/AKT pathway is relevant for MPA-independent tumor growth. Three-dimensional cultures were useful to test the effects of kinase inhibitors on breast cancer growth and highlight the need for in vivo models to validate experimental tools used for selective therapeutic targeting.
Conflict of interest statement
Figures


Similar articles
-
Combined inhibition of MEK and PI3K pathways overcomes acquired resistance to EGFR-TKIs in non-small cell lung cancer.Cancer Sci. 2018 Oct;109(10):3183-3196. doi: 10.1111/cas.13763. Epub 2018 Sep 14. Cancer Sci. 2018. PMID: 30098066 Free PMC article.
-
Activation of PI3K/Akt/mTOR signaling in the tumor stroma drives endocrine therapy-dependent breast tumor regression.Oncotarget. 2015 Sep 8;6(26):22081-97. doi: 10.18632/oncotarget.4203. Oncotarget. 2015. PMID: 26098779 Free PMC article.
-
Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways.Int J Cancer. 2010 Jan 15;126(2):545-62. doi: 10.1002/ijc.24750. Int J Cancer. 2010. PMID: 19609946
-
Targeting the PI3K/AKT/mTOR Pathway in Hormone-Positive Breast Cancer.Drugs. 2020 Nov;80(16):1685-1697. doi: 10.1007/s40265-020-01394-w. Drugs. 2020. PMID: 32894420 Free PMC article. Review.
-
An update on promising and emerging protein kinase B/AKT inhibitors for breast cancer.Expert Opin Pharmacother. 2025 Feb;26(3):235-247. doi: 10.1080/14656566.2025.2454290. Epub 2025 Jan 27. Expert Opin Pharmacother. 2025. PMID: 39846444 Review.
Cited by
-
The resistance of intracellular mediators to doxorubicin and cisplatin are distinct in 3D and 2D endometrial cancer.J Transl Med. 2012 Mar 6;10:38. doi: 10.1186/1479-5876-10-38. J Transl Med. 2012. PMID: 22394685 Free PMC article.
-
hMENA(11a) contributes to HER3-mediated resistance to PI3K inhibitors in HER2-overexpressing breast cancer cells.Oncogene. 2016 Feb 18;35(7):887-96. doi: 10.1038/onc.2015.143. Epub 2015 May 11. Oncogene. 2016. PMID: 25961924
-
Antiprogestins in gynecological diseases.Reproduction. 2015 Jan;149(1):R15-33. doi: 10.1530/REP-14-0416. Epub 2014 Sep 24. Reproduction. 2015. PMID: 25252652 Free PMC article. Review.
-
Unravelling the antimetastatic potential of pentoxifylline, a methylxanthine derivative in human MDA-MB-231 breast cancer cells.Mol Cell Biochem. 2011 Dec;358(1-2):141-51. doi: 10.1007/s11010-011-0929-8. Epub 2011 Jul 3. Mol Cell Biochem. 2011. PMID: 21725843
-
Ligand-free estrogen receptor activity complements IGF1R to induce the proliferation of the MCF-7 breast cancer cells.BMC Cancer. 2012 Jul 16;12:291. doi: 10.1186/1471-2407-12-291. BMC Cancer. 2012. PMID: 22799881 Free PMC article.
References
-
- Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. - PubMed
-
- Robertson JF. Fulvestrant (Faslodex)—how to make a good drug better. Oncologist. 2007;12:774–784. - PubMed
-
- Macedo LF, Sabnis GJ, Goloubeva OG, Brodie A. Combination of anastrozole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res. 2008;68:3516–3522. - PubMed
-
- Klijn JG, de Jong FH, Bakker GH, Lamberts SW, Rodenburg CJ, et al. Antiprogestins, a new form of endocrine therapy for human breast cancer. Cancer Res. 1989;49:2851–2856. - PubMed
-
- Liang Y, Besch-Williford C, Brekken RA, Hyder SM. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating antitumor therapeutics. Cancer Res. 2007;67:9929–9936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

