Heterozygosity for Pten promotes tumorigenesis in a mouse model of medulloblastoma
- PMID: 20520772
- PMCID: PMC2877103
- DOI: 10.1371/journal.pone.0010849
Heterozygosity for Pten promotes tumorigenesis in a mouse model of medulloblastoma
Abstract
Background: Recent publications have described an important role for cross talk between PI-3 kinase and sonic hedgehog signaling pathways in the pathogenesis of medulloblastoma.
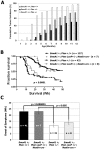
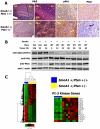
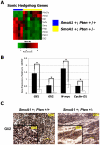
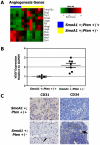
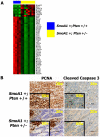
Methodology/principal findings: We crossed mice with constitutive activation of Smoothened, SmoA1, with Pten deficient mice. Both constitutive and conditional Pten deficiency doubled the incidence of mice with symptoms of medulloblastoma and resulted in decreased survival. Analysis revealed a clear separation of gene signatures, with up-regulation of genes in the PI-3 kinase signaling pathway, including downstream activation of angiogenesis in SmoA1+/-; Pten +/- medulloblastomas. Western blotting and immunohistochemistry confirmed reduced or absent Pten, Akt activation, and increased angiogenesis in Pten deficient tumors. Down-regulated genes included genes in the sonic hedgehog pathway and tumor suppressor genes. SmoA1+/-; Pten +/+ medulloblastomas appeared classic in histology with increased proliferation and diffuse staining for apoptosis. In contrast, Pten deficient tumors exhibited extensive nodularity with neuronal differentiation separated by focal areas of intense staining for proliferation and virtually absent apoptosis. Examination of human medulloblastomas revealed low to absent PTEN expression in over half of the tumors. Kaplan-Meier analysis confirmed worse overall survival in patients whose tumor exhibited low to absent PTEN expression.
Conclusions/significance: This suggests that PTEN expression is a marker of favorable prognosis and mouse models with activation of PI-3 kinase pathways may be important tools for preclinical evaluation of promising agents for the treatment of medulloblastoma.
Conflict of interest statement
Figures

Similar articles
-
Phosphatidylinositol 3'-kinase/AKT signaling is activated in medulloblastoma cell proliferation and is associated with reduced expression of PTEN.Clin Cancer Res. 2006 May 15;12(10):3019-27. doi: 10.1158/1078-0432.CCR-05-2187. Clin Cancer Res. 2006. PMID: 16707597
-
PI-3K Inhibitors Preferentially Target CD15+ Cancer Stem Cell Population in SHH Driven Medulloblastoma.PLoS One. 2016 Mar 3;11(3):e0150836. doi: 10.1371/journal.pone.0150836. eCollection 2016. PLoS One. 2016. PMID: 26938241 Free PMC article.
-
The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas.Cancer Res. 2004 Nov 1;64(21):7794-800. doi: 10.1158/0008-5472.CAN-04-1813. Cancer Res. 2004. PMID: 15520185
-
ASC deficiency suppresses proliferation and prevents medulloblastoma incidence.Oncogene. 2015 Jan 15;34(3):394-402. doi: 10.1038/onc.2013.577. Epub 2014 Jan 27. Oncogene. 2015. PMID: 24469054 Free PMC article.
-
Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 2: PI3K/Akt/PTEN, mTOR, SHH/PTCH and angiogenesis.Expert Rev Anticancer Ther. 2004 Feb;4(1):105-28. doi: 10.1586/14737140.4.1.105. Expert Rev Anticancer Ther. 2004. PMID: 14748662 Review.
Cited by
-
An animal model of MYC-driven medulloblastoma.Cancer Cell. 2012 Feb 14;21(2):155-67. doi: 10.1016/j.ccr.2011.12.021. Cancer Cell. 2012. PMID: 22340590 Free PMC article.
-
Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: new therapeutic opportunities.Nat Med. 2013 Nov;19(11):1518-23. doi: 10.1038/nm.3328. Epub 2013 Sep 29. Nat Med. 2013. PMID: 24076665 Free PMC article.
-
Response to bevacizumab, irinotecan, and temozolomide in children with relapsed medulloblastoma: a multi-institutional experience.Childs Nerv Syst. 2013 Apr;29(4):589-96. doi: 10.1007/s00381-012-2013-4. Epub 2013 Jan 8. Childs Nerv Syst. 2013. PMID: 23296323 Free PMC article.
-
Medulloblastoma development: tumor biology informs treatment decisions.CNS Oncol. 2015;4(2):79-89. doi: 10.2217/cns.14.58. CNS Oncol. 2015. PMID: 25768332 Free PMC article. Review.
-
BAI1 Suppresses Medulloblastoma Formation by Protecting p53 from Mdm2-Mediated Degradation.Cancer Cell. 2018 Jun 11;33(6):1004-1016.e5. doi: 10.1016/j.ccell.2018.05.006. Cancer Cell. 2018. PMID: 29894688 Free PMC article.
References
-
- Rood BR, Macdonald TJ, Packer RJ. Current treatment of medulloblastoma: recent advances and future challenges. Semin Oncol. 2004;31:666–675. - PubMed
-
- Giangaspero F BS, Giordana MT, Kleihues P, Trojanowski JQ. Medulloblastoma; In: Kleihues PCW, editor. Lyon, France: International Agency for Research of Cancer; 2000. pp. 96–103.
-
- Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. - PubMed
-
- Garre ML, Cama A, Bagnasco F, Morana G, Giangaspero F, et al. Medulloblastoma variants: age-dependent occurrence and relation to Gorlin syndrome–a new clinical perspective. Clin Cancer Res. 2009;15:2463–2471. - PubMed
-
- Packer RJ, Rood BR, MacDonald TJ. Medulloblastoma: present concepts of stratification into risk groups. Pediatr Neurosurg. 2003;39:60–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

