Preferential amplification of CD8 effector-T cells after transcutaneous application of an inactivated influenza vaccine: a randomized phase I trial
- PMID: 20520820
- PMCID: PMC2877091
- DOI: 10.1371/journal.pone.0010818
Preferential amplification of CD8 effector-T cells after transcutaneous application of an inactivated influenza vaccine: a randomized phase I trial
Abstract
Background: Current conventional vaccination approaches do not induce potent CD8 T-cell responses for fighting mostly variable viral diseases such as influenza, avian influenza viruses or HIV. Following our recent study on vaccine penetration by targeting of vaccine to human hair follicular ducts surrounded by Langerhans cells, we tested in the first randomized Phase-Ia trial based on hair follicle penetration (namely transcutaneous route) the induction of virus-specific CD8 T cell responses.
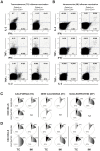
Methods and findings: We chose the inactivated influenza vaccine - a conventional licensed tetanus/influenza (TETAGRIP) vaccine - to compare the safety and immunogenicity of transcutaneous (TC) versus IM immunization in two randomized controlled, multi-center Phase I trials including 24 healthy-volunteers and 12 HIV-infected patients. Vaccination was performed by application of inactivated influenza vaccine according to a standard protocol allowing the opening of the hair duct for the TC route or needle-injection for the IM route. We demonstrated that the safety of the two routes was similar. We showed the superiority of TC application, but not the IM route, to induce a significant increase in influenza-specific CD8 cytokine-producing cells in healthy-volunteers and in HIV-infected patients. However, these routes did not differ significantly for the induction of influenza-specific CD4 responses, and neutralizing antibodies were induced only by the IM route. The CD8 cell response is thus the major immune response observed after TC vaccination.
Conclusions: This Phase Ia clinical trial (Manon05) testing an anti-influenza vaccine demonstrated that vaccines designed for antibody induction by the IM route, generate vaccine-specific CD8 T cells when administered transcutaneously. These results underline the necessity of adapting vaccination strategies to control complex infectious diseases when CD8 cellular responses are crucial. Our work opens up a key area for the development of preventive and therapeutic vaccines for diseases in which CD8 cells play a crucial role.
Trial registration: Clinicaltrials.gov NCT00261001.
Conflict of interest statement
Figures


References
-
- Wharton M, Strikas RA, Harpaz R, Rotz LD, Schwartz B, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1–16. - PubMed
-
- Patterson S, Papagatsias T, Benlahrech A. Use of adenovirus in vaccines for HIV. Handb Exp Pharmacol. 2009:275–293. - PubMed
-
- White A. Why vaccines are not the answer - the failure of V520 and the importance of cell-mediated immunity in the fight against HIV. Med Hypotheses. 2008;71:909–913. - PubMed
-
- Doherty PC, Hou S, Tripp RA. CD8+ T-cell memory to viruses. Curr Opin Immunol. 1994;6:545–552. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

