The monofunctional catalase KatE of Xanthomonas axonopodis pv. citri is required for full virulence in citrus plants
- PMID: 20520822
- PMCID: PMC2875408
- DOI: 10.1371/journal.pone.0010803
The monofunctional catalase KatE of Xanthomonas axonopodis pv. citri is required for full virulence in citrus plants
Abstract
Background: Xanthomonas axonopodis pv. citri (Xac) is an obligate aerobic phytopathogen constantly exposed to hydrogen peroxide produced by normal aerobic respiration and by the plant defense response during plant-pathogen interactions. Four putative catalase genes have been identified in silico in the Xac genome, designated as katE, catB, srpA (monofunctional catalases) and katG (bifunctional catalase).
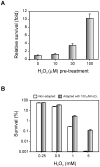
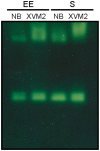
Methodology/principal findings: Xac catalase activity was analyzed using native gel electrophoresis and semi-quantitative RT-PCR. We demonstrated that the catalase activity pattern was regulated in different growth stages displaying the highest levels during the stationary phase. KatE was the most active catalase in this phase of growth. At this stage cells were more resistant to hydrogen peroxide as was determined by the analysis of CFU after the exposition to different H(2)O(2) concentrations. In addition, Xac exhibited an adaptive response to hydrogen peroxide, displaying higher levels of catalase activity and H(2)O(2) resistance after treatment with sub-lethal concentrations of the oxidant. In the plant-like medium XVM2 the expression of KatE was strongly induced and in this medium Xac was more resistant to H(2)O(2). A XackatE mutant strain was constructed by insertional mutagenesis. We observed that catalase induction in stationary phase was lost meanwhile the adaptive response to peroxide was maintained in this mutant. Finally, the XackatE strain was assayed in planta during host plant interaction rendering a less aggressive phenotype with a minor canker formation.
Conclusions: Our results confirmed that in contrast to other Xanthomonas species, Xac catalase-specific activity is induced during the stationary phase of growth in parallel with the bacterial resistance to peroxide challenge. Moreover, Xac catalases expression pattern is modified in response to any stimuli associated with the plant or the microenvironment it provides. The catalase KatE has been shown to have an important function for the colonization and survival of the bacterium in the citrus plant during the pathogenic process. Our work provides the first genetic evidence to support a monofunctional catalase as a virulence factor in Xac.
Conflict of interest statement
Figures


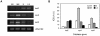
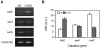

Similar articles
-
Modifications of Xanthomonas axonopodis pv. citri lipopolysaccharide affect the basal response and the virulence process during citrus canker.PLoS One. 2012;7(7):e40051. doi: 10.1371/journal.pone.0040051. Epub 2012 Jul 6. PLoS One. 2012. PMID: 22792211 Free PMC article.
-
A LOV protein modulates the physiological attributes of Xanthomonas axonopodis pv. citri relevant for host plant colonization.PLoS One. 2012;7(6):e38226. doi: 10.1371/journal.pone.0038226. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675525 Free PMC article.
-
KatG, the Bifunctional Catalase of Xanthomonas citri subsp. citri, Responds to Hydrogen Peroxide and Contributes to Epiphytic Survival on Citrus Leaves.PLoS One. 2016 Mar 18;11(3):e0151657. doi: 10.1371/journal.pone.0151657. eCollection 2016. PLoS One. 2016. PMID: 26990197 Free PMC article.
-
A novel two-component response regulator links rpf with biofilm formation and virulence of Xanthomonas axonopodis pv. citri.PLoS One. 2013 Apr 23;8(4):e62824. doi: 10.1371/journal.pone.0062824. Print 2013. PLoS One. 2013. PMID: 23626857 Free PMC article.
-
The Structural Biology of Catalase Evolution.Subcell Biochem. 2024;104:33-47. doi: 10.1007/978-3-031-58843-3_3. Subcell Biochem. 2024. PMID: 38963482 Review.
Cited by
-
Evaluation of the virulence of Xanthomonas campestris pv. campestris mutant strains lacking functional genes in the OxyR regulon.Curr Microbiol. 2011 Aug;63(2):232-7. doi: 10.1007/s00284-011-9970-9. Epub 2011 Jun 28. Curr Microbiol. 2011. PMID: 21710133
-
Structural-functional characterization and physiological significance of ferredoxin-NADP reductase from Xanthomonas axonopodis pv. citri.PLoS One. 2011;6(11):e27124. doi: 10.1371/journal.pone.0027124. Epub 2011 Nov 9. PLoS One. 2011. PMID: 22096528 Free PMC article.
-
The periplasmic binding protein NrtT affects xantham gum production and pathogenesis in Xanthomonas citri.FEBS Open Bio. 2017 Sep 13;7(10):1499-1514. doi: 10.1002/2211-5463.12281. eCollection 2017 Oct. FEBS Open Bio. 2017. PMID: 28979839 Free PMC article.
-
KatE From the Bacterial Plant Pathogen Ralstonia solanacearum Is a Monofunctional Catalase Controlled by HrpG That Plays a Major Role in Bacterial Survival to Hydrogen Peroxide.Front Plant Sci. 2020 Jul 31;11:1156. doi: 10.3389/fpls.2020.01156. eCollection 2020. Front Plant Sci. 2020. PMID: 32849714 Free PMC article.
-
Comparative genomics of 43 strains of Xanthomonas citri pv. citri reveals the evolutionary events giving rise to pathotypes with different host ranges.BMC Genomics. 2015 Dec 23;16:1098. doi: 10.1186/s12864-015-2310-x. BMC Genomics. 2015. PMID: 26699528 Free PMC article.
References
-
- Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. - PubMed
-
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049–6055. - PubMed
-
- Green J, Paget MS. Bacterial redox sensors. Nat Rev Microbiol. 2004;2:954–966. - PubMed
-
- Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

