Three repeat isoforms of tau inhibit assembly of four repeat tau filaments
- PMID: 20520830
- PMCID: PMC2876030
- DOI: 10.1371/journal.pone.0010810
Three repeat isoforms of tau inhibit assembly of four repeat tau filaments
Abstract
Tauopathies are defined by assembly of the microtubule associated protein tau into filamentous tangles and classified by the predominant tau isoform within these aggregates. The major isoforms are determined by alternative mRNA splicing of exon 10 generating tau with three (3R) or four (4R) approximately 32 amino acid imperfect repeats in the microtubule binding domain. In normal adult brains there is an approximately equimolar ratio of 3R and 4R tau which is altered by several disease-causing mutations in the tau gene. We hypothesized that when 4R and 3R tau isoforms are not at equimolar ratios aggregation is favored. Here we provide evidence for the first time that the combination of 3R and 4R tau isoforms results in less in vitro heparin induced polymerization than with 4R preparations alone. This effect was independent of reducing conditions and the presence of alternatively spliced exons 2 and 3 N-terminal inserts. The addition of even small amounts of 3R to 4R tau assembly reactions significantly decreased 4R assembly. Together these findings suggest that co-expression of 3R and 4R tau isoforms reduce tau filament assembly and that 3R tau isoforms inhibit 4R tau assembly. Expression of equimolar amounts of 3R and 4R tau in adult humans may be necessary to maintain proper neuronal microtubule dynamics and to prevent abnormal tau filament assembly. Importantly, these findings indicate that disruption of the normal equimolar 3R to 4R ratio may be sufficient to drive tau aggregation and that restoration of the tau isoform balance may have important therapeutic implications in tauopathies.
Conflict of interest statement
Figures

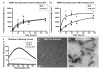
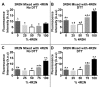
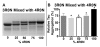
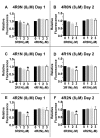
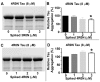

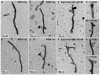
References
-
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. - PubMed
-
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. - PubMed
-
- Andreadis A, Brown WM, Kosi KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–10633. - PubMed
-
- Kosik KS, Orecchio LD, Bakalis S, Neve RL. Developmentally regulated expression of specific tau sequences. Neuron. 1989;2:1389–1397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

