Electrophilic aromatic selenylation: new OPRT inhibitors
- PMID: 20521773
- PMCID: PMC2906230
- DOI: 10.1021/ol1010032
Electrophilic aromatic selenylation: new OPRT inhibitors
Abstract
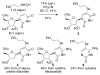
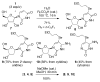
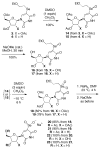
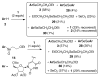
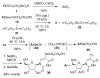
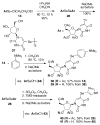
2-Ethoxyethaneseleninic acid reacts with electron-rich aromatic substrates to deliver, by way of the selenoxides, the (2-ethoxyethyl)seleno ethers, which can in turn be transformed into a diverse set of aryl-selenylated products. Among these, a family of 5-uridinyl derivatives shows submicromolar inhibition of human and malarial orotate phosphoribosyltransferase.
Figures
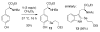
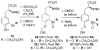
References
-
- Iwaoka M, Tomoda S. Top Curr Chem. 2000;208:55–80.
- Wirth T. Top Curr Chem. 2000;208:1–5.
- Back TG. Organoselenium Chemistry – A Practical Approach. Oxford Press; New York: 2000.
-
- Mugesh G, du Mont WW, Sies H. Chem Rev. 2001;101:2125–2179. - PubMed
- Soriano-Garcia M. Curr Med Chem. 2001;11:1657–1669. - PubMed
- Armishaw CJ, Daly NL, Nevin ST, Adams DJ, Craik DJ, Alewood PF. J Biol Chem. 2006;281:14136–14143. - PubMed
- Abdo M, Liu S, Zhou B, Walls CD, Knapp S, Zhang ZY. J Am Chem Soc. 2008;130:13196–13197. - PubMed
- Desai D, Madhunapantula SV, Gowdahalli K, Sharma A, Chandagaludoreswamy R, El-Bayoumy K, Robertson GP, Amin S. Bioorg Med Chem Lett. 2010;20:2038–2043. and references cited therein. - PMC - PubMed
-
- Abdo M, Knapp S. J Am Chem Soc. 2008;130:9234–9235. - PubMed
-
-
Electrophilic aryl selenylations: (phenols) Oddershede J, Henriksen L, Larsen S. Org Biomol Chem. 2003;1:1053–1060.and references cited therein. (indoles) Crich D, Davies JW. Tetrahedron Lett. 1989;30:4307–4308.and references cited therein.
-
-
-
Other uridine selenylations: Choi S, Kalman TI, Bardos TJ. J Med Chem. 1979;22:618–621.Schinazi R, Arbiser J, Lee J, Kalman T, Prusoff W. J Med Chem. 1986;29:1293–1295.Lee CH, Kim YH. Tetrahedron Lett. 1991;32:2401–2404.Goudgaon NM, Naguib FNM, el Kouni MH, Schinazi RF. J Med Chem. 1993;36:4250–4254.Roh KR, Chang HK, Kim YH. Heterocycles. 1998;48:437–4441.Hassan AEA, Sheng J, Jiang J, Zhang W, Huang Z. Org Lett. 2009;11:2503–2506.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

