AMPK inhibition in health and disease
- PMID: 20522000
- PMCID: PMC3132561
- DOI: 10.3109/10409238.2010.488215
AMPK inhibition in health and disease
Abstract
All living organisms depend on dynamic mechanisms that repeatedly reassess the status of amassed energy, in order to adapt energy supply to demand. The AMP-activated protein kinase (AMPK) alphabetagamma heterotrimer has emerged as an important integrator of signals managing energy balance. Control of AMPK activity involves allosteric AMP and ATP regulation, auto-inhibitory features and phosphorylation of its catalytic (alpha) and regulatory (beta and gamma) subunits. AMPK has a prominent role not only as a peripheral sensor but also in the central nervous system as a multifunctional metabolic regulator. AMPK represents an ideal second messenger for reporting cellular energy state. For this reason, activated AMPK acts as a protective response to energy stress in numerous systems. However, AMPK inhibition also actively participates in the control of whole body energy homeostasis. In this review, we discuss recent findings that support the role and function of AMPK inhibition under physiological and pathological states.
Conflict of interest statement
Figures
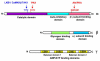
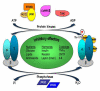

References
-
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. - PubMed
-
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. S6 Kinase Deletion Suppresses Muscle Growth Adaptations to Nutrient Availability by Activating AMP Kinase. Cell metabolism. 2007;5:476–487. - PubMed
-
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
