Secretion of the chlamydial virulence factor CPAF requires the Sec-dependent pathway
- PMID: 20522495
- PMCID: PMC3068695
- DOI: 10.1099/mic.0.040527-0
Secretion of the chlamydial virulence factor CPAF requires the Sec-dependent pathway
Abstract
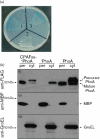
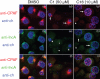
The chlamydial protease/proteasome-like activity factor (CPAF) is secreted into the host cytosol to degrade various host factors that benefit chlamydial intracellular survival. Although the full-length CPAF is predicted to contain a putative signal peptide at its N terminus, the secretion pathway of CPAF is still unknown. Here, we have provided experimental evidence that the N-terminal sequence covering the M1-G31 region was cleaved from CPAF during chlamydial infection. The CPAF N-terminal sequence, when expressed in a phoA gene fusion construct, was able to direct the export of the mature PhoA protein across the inner membrane of wild-type Escherichia coli. However, E. coli mutants deficient in SecB failed to support the CPAF signal-peptide-directed secretion of PhoA. Since native PhoA secretion was known to be independent of SecB, this SecB dependence must be rendered by the CPAF leader peptide. Furthermore, lack of SecY function also blocked the CPAF signal-peptide-directed secretion of PhoA. Most importantly, CPAF secretion into the host cell cytosol during chlamydial infection was selectively inhibited by an inhibitor specifically targeting type I signal peptidase but not by a type III secretion-system-specific inhibitor. Together, these observations have demonstrated that the chlamydial virulence factor CPAF relies on Sec-dependent transport for crossing the chlamydial inner membrane, which has provided essential information for further delineating the pathways of CPAF action and understanding chlamydial pathogenic mechanisms.
Figures


Similar articles
-
Localization of Chlamydia trachomatis hypothetical protein CT311 in host cell cytoplasm.Microb Pathog. 2011 Sep;51(3):101-9. doi: 10.1016/j.micpath.2011.05.002. Epub 2011 May 13. Microb Pathog. 2011. PMID: 21605656 Free PMC article.
-
[Interaction of SecB and SecA with the N-terminal region of mature alkaline phosphatase on its secretion in Escherichia coli].Mol Biol (Mosk). 2003 Jul-Aug;37(4):712-8. Mol Biol (Mosk). 2003. PMID: 12942645 Russian.
-
Autoprocessing and self-activation of the secreted protease CPAF in Chlamydia-infected cells.Microb Pathog. 2010 Oct;49(4):164-73. doi: 10.1016/j.micpath.2010.05.008. Epub 2010 May 25. Microb Pathog. 2010. PMID: 20510344 Free PMC article.
-
Conserved type III secretion system exerts important roles in Chlamydia trachomatis.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5404-14. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337183 Free PMC article. Review.
-
A path forward for the chlamydial virulence factor CPAF.Microbes Infect. 2013 Dec;15(14-15):1026-32. doi: 10.1016/j.micinf.2013.09.008. Epub 2013 Oct 18. Microbes Infect. 2013. PMID: 24141088 Free PMC article. Review.
Cited by
-
A Chlamydial Plasmid-Dependent Secretion System for the Delivery of Virulence Factors to the Host Cytosol.mBio. 2021 Jun 29;12(3):e0117921. doi: 10.1128/mBio.01179-21. Epub 2021 Jun 8. mBio. 2021. PMID: 34101486 Free PMC article.
-
Resistance to a novel antichlamydial compound is mediated through mutations in Chlamydia trachomatis secY.Antimicrob Agents Chemother. 2012 Aug;56(8):4296-302. doi: 10.1128/AAC.00356-12. Epub 2012 May 29. Antimicrob Agents Chemother. 2012. PMID: 22644029 Free PMC article.
-
Secretory pathway of cellulase: a mini-review.Biotechnol Biofuels. 2013 Dec 2;6(1):177. doi: 10.1186/1754-6834-6-177. Biotechnol Biofuels. 2013. PMID: 24295495 Free PMC article.
-
Chlamydia trachomatis secretion of proteases for manipulating host signaling pathways.Front Microbiol. 2011 Feb 8;2:14. doi: 10.3389/fmicb.2011.00014. eCollection 2011. Front Microbiol. 2011. PMID: 21687409 Free PMC article.
-
Chlamydia trachomatis secretion of hypothetical protein CT622 into host cell cytoplasm via a secretion pathway that can be inhibited by the type III secretion system inhibitor compound 1.Microbiology (Reading). 2011 Apr;157(Pt 4):1134-1144. doi: 10.1099/mic.0.047746-0. Epub 2011 Jan 13. Microbiology (Reading). 2011. PMID: 21233161 Free PMC article.
References
-
- Betts, H. J., Wolf, K. & Fields, K. A. (2009). Effector protein modulation of host cells: examples in the Chlamydia spp. arsenal. Curr Opin Microbiol 12, 81–87. - PubMed
-
- Bhatnagar, R. & Batra, S. (2001). Anthrax toxin. Crit Rev Microbiol 27, 167–200. - PubMed
-
- Campbell, L. A. & Kuo, C. C. (2004). Chlamydia pneumoniae – an infectious risk factor for atherosclerosis? Nat Rev Microbiol 2, 23–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

