Improvement in growth after 1 year of growth hormone therapy in well-nourished infants with growth retardation secondary to chronic renal failure: results of a multicenter, controlled, randomized, open clinical trial
- PMID: 20522533
- PMCID: PMC2893059
- DOI: 10.2215/CJN.07791109
Improvement in growth after 1 year of growth hormone therapy in well-nourished infants with growth retardation secondary to chronic renal failure: results of a multicenter, controlled, randomized, open clinical trial
Abstract
Background and objectives: Our aim was to evaluate the growth-promoting effect of growth hormone (GH) treatment in infants with chronic renal failure (CRF) and persistent growth retardation despite adequate nutritional and metabolic management.
Design, setting, participants, & measurements: The study design included randomized, parallel groups in an open, multicenter trial comparing GH (0.33 mg/kg per wk) with nontreatment with GH during 12 months. Sixteen infants who had growth retardation, were aged 12+/-3 months, had CRF (GFR<or=60 ml/min per 1.73 m2), and had adequate nutritional intake and good metabolic control were recruited from eight pediatric nephrology departments from Spain and Portugal. Main outcome measures were body length, body weight, bone age, biochemical and hormonal analyses, renal function, bone mass, and adverse effects.
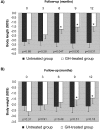
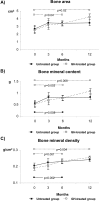
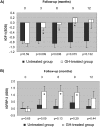
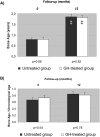
Results: Length gain in infants who were treated with GH was statistically greater (P<0.05) than that of nontreated children (14.5 versus 9.5 cm/yr; SD score 1.43 versus -0.11). The GH-induced stimulation of growth was associated with no undesirable effects on bone maturation, renal failure progression, or metabolic control. In addition, GH treatment improved forearm bone mass and increased serum concentrations of total and free IGF-I and IGF-binding protein 3 (IGFBP-3), whereas IGF-II, IGFBP-1, IGFBP-2, GH-binding protein, ghrelin, and leptin were not modified.
Conclusions: Infants with CRF and growth retardation despite good metabolic and nutritional control benefit from GH treatment without adverse effects during 12 months of therapy.
Trial registration: ClinicalTrials.gov NCT00184769.
Figures
References
-
- NAPRTCS: NAPRTCS 2008 Annual Report. Available at: http://spitfire.emmes.com/study/ped/index.htm Accessed June 3, 2009
-
- Furth SL: Growth and nutrition in children with chronic kidney disease. Adv Chronic Kidney Dis 12: 366–371, 2005 - PubMed
-
- Kuizon BD, Salusky IB: Growth retardation in children with chronic renal failure. J Bone Miner Res 14: 1680–1690, 1999 - PubMed
-
- Karlberg J, Schaefer F, Hennicke M, Wingen AM, Rigden S, Mehls O: Early age-dependent growth impairment in chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. Pediatr Nephrol 10: 283–287, 1996 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

