A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients
- PMID: 20522535
- PMCID: PMC2924413
- DOI: 10.2215/CJN.09421209
A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients
Abstract
Background and objectives: Hemodialysis patients (HD) display high rates of cardiac diseases and mortality. In chronic kidney disease, vascular injury leads to coronary artery disease, heart failure, and stroke. Carotid intima-media thickness (CIMT) measurements are currently widely used in randomized controlled trials (RCTs) to study the efficacy of interventions. An RCT was designed for the assessment of the safety and effectiveness of spironolactone to inhibit the progression of CIMT in HD patients as a primary outcome. Secondary outcomes included measurements of plasma potassium.
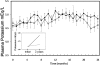
Design, setting, participants, & measurements: HD patients were randomly assigned to receive 50 mg spironolactone or placebo thrice weekly after dialysis. In between dialysis sessions, plasma potassium concentrations were measured every month. Ultrasonographic measurements of CIMT were done at the beginning of the study and after 2 years.
Results: Fifty-three age- and sex-adjusted patients (30 with drug and 23 with placebo) successfully completed the trial. There were no significant differences between the two groups in all profiles studied at baseline. Measurements of CIMT after 2 years showed a progression in the placebo group, whereas in the spironolactone group a significant decrease or even reversed CIMT was observed. Progression rates (mm/yr) were: common carotid, placebo: 0.06 +/- 0.07, spironolactone: 0.01 +/- 0.04; carotid bifurcation, placebo: 0.15 +/- 0.27, spironolactone: 0.0001 +/- 0.01; internal carotid, placebo: 0.10 +/- 0.12, spironolactone: -0.10 +/- 0.15. No episodes of hyperkalemia were observed, but a slight increase in plasma potassium was found in the spironolactone group.
Conclusions: Fifty milligrams of spironolactone thrice weekly significantly reduced the progression of CIMT in HD patients.
Figures


References
-
- London GM, Marchais SJ, Metivier F, Guerin AP. Cardiovascular risk in end-stage renal disease: Vascular aspects. Nephrol Dial Transplant 15 [Suppl 5]: 97–104, 2007 - PubMed
-
- Schiffrin EL, Lipman ML, Mann JFE: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 - PubMed
-
- Ma KW, Greene EL, Raij L: Cardiovascular risk factors in chronic renal failure and hemodialysis populations. Am J Kidney Dis 19: 505–513, 1992 - PubMed
-
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JFE, Motolesem M, Ponticelli C, Ritz E, Zucchelli P: Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med 334: 939–945, 1996 - PubMed
-
- Wolf G, Ritz E: Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: Pathophysiology and indications. Kidney Int 67: 799–812, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

