Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria
- PMID: 20522536
- PMCID: PMC2924405
- DOI: 10.2215/CJN.09321209
Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria
Abstract
Background and objectives: This report summarizes the first phase 1 trial treating patients with microalbuminuric diabetic kidney disease (DKD) using FG-3019, a human monoclonal antibody to connective tissue growth factor (CTGF). CTGF is critically involved in processes of progressive fibrosis, including DKD. This phase 1, open-label, dose-escalation trial evaluated safety, pharmacokinetics, and possible therapeutic effects of FG-3019 on albuminuria, proteinuria, and tubular proteins.
Design, setting, participants, and measurements: Microalbuminuric subjects (n = 24) with type 2 (79%) or type 1 (21%) diabetes received 3 or 10 mg/kg FG-3019 dosed intravenously every 14 days for four doses. Albuminuria and safety follow-up were to days 62 and 365, respectively.
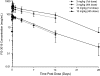
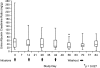
Results: No infusion was interrupted for symptoms, although 5 of 24 subjects had mild infusion-day adverse events thought to be possibly drug-related. No subject developed anti-FG-3019 antibodies. FG-3019 clearance was lower at 10 mg/kg than at 3 mg/kg, suggesting a saturable elimination pathway. Although this study was not designed for efficacy testing, it was notable that urinary albumin/creatinine ratio (ACR) decreased significantly from mean pretreatment ACR of 48 mg/g to mean post-treatment (day 56) ACR of 20 mg/g (P = 0.027) without evidence for a dose-response relationship.
Conclusions: Treatment of microalbuminuric DKD subjects using FG-3019 was well tolerated and associated with a decrease in albuminuria. The data demonstrate a saturable pathway for drug elimination, minimal infusion adverse events, and no significant drug-attributable adverse effects over the year of follow-up. Changes in albuminuria were promising but require validation in a prospective, randomized, blinded study.
Figures
References
-
- U.S. Renal Data System: USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006
-
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 865–869, 2001 - PubMed
-
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 - PubMed
-
- Ruggenenti P, Pagano E, Tammuzzo L, Benini R, Garattini L, Remuzzi G: Ramipril prolongs life and is cost effective in chronic proteinuric nephropathies. Kidney Int 59: 286–294, 2001 - PubMed
-
- Wahab NA, Schaefer L, Weston BS, Yiannikouris O, Wright A, Babelova A, Schaefer R, Mason RM: Glomerular expression of thrombospondin-1, TGF-beta and CTGF at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetalogica 48: 2650–2660, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

