Iron and porphyrin trafficking in heme biogenesis
- PMID: 20522548
- PMCID: PMC2930673
- DOI: 10.1074/jbc.R110.119503
Iron and porphyrin trafficking in heme biogenesis
Abstract
Iron is an essential element for diverse biological functions. In mammals, the majority of iron is enclosed within a single prosthetic group: heme. In metazoans, heme is synthesized via a highly conserved and coordinated pathway within the mitochondria. However, iron is acquired from the environment and subsequently assimilated into various cellular pathways, including heme synthesis. Both iron and heme are toxic but essential cofactors. How is iron transported from the extracellular milieu to the mitochondria? How are heme and heme intermediates coordinated with iron transport? Although recent studies have answered some questions, several pieces of this intriguing puzzle remain unsolved.
Figures

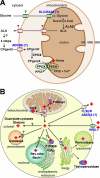
References
-
- Krewulak K. D., Vogel H. J. (2008) Biochim. Biophys. Acta 1778, 1781–1804 - PubMed
-
- Hentze M. W., Muckenthaler M. U., Andrews N. C. (2004) Cell 117, 285–297 - PubMed
-
- De Domenico I., McVey Ward D., Kaplan J. (2008) Nat. Rev. Mol. Cell Biol. 9, 72–81 - PubMed
-
- Simpson R. J., McKie A. T. (2009) Cell Metab. 10, 84–87 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

