Mesenchymal bone morphogenetic protein signaling is required for normal pancreas development
- PMID: 20522595
- PMCID: PMC2911072
- DOI: 10.2337/db09-1010
Mesenchymal bone morphogenetic protein signaling is required for normal pancreas development
Abstract
Objective: Pancreas organogenesis is orchestrated by interactions between the epithelium and the mesenchyme, but these interactions are not completely understood. Here we investigated a role for bone morphogenetic protein (BMP) signaling within the pancreas mesenchyme and found it to be required for the normal development of the mesenchyme as well as for the pancreatic epithelium.
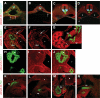
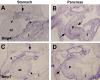
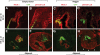
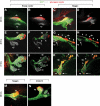
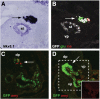
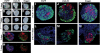
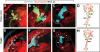
Research design and methods: We analyzed active BMP signaling by immunostaining for phospho-Smad1,5,8 and tested whether pancreas development was affected by BMP inhibition after expression of Noggin and dominant negative BMP receptors in chicken and mouse pancreas.
Results: Endogenous BMP signaling is confined to the mesenchyme in the early pancreas and inhibition of BMP signaling results in severe pancreatic hypoplasia with reduced epithelial branching. Notably, we also observed an excessive endocrine differentiation when mesenchymal BMP signaling is blocked, presumably secondary to defective mesenchyme to epithelium signaling.
Conclusions: We conclude that BMP signaling plays a previously unsuspected role in the mesenchyme, required for normal development of the mesenchyme as well as for the epithelium.
Figures

References
-
- Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J: An illustrated review of early pancreas development in the mouse. Endocr Rev 2007;28:685–705 - PubMed
-
- Kumar M, Jordan N, Melton D, Grapin-Botton A: Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol 2003;259:109–122 - PubMed
-
- Lammert E, Cleaver O, Melton D: Induction of pancreatic differentiation by signals from blood vessels. Science 2001;294:564–567 - PubMed
-
- Kim SK, Hebrok M, Melton DA: Pancreas development in the chick embryo. Cold Spring Harb Symp Quant Biol 1997;62:377–383 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

