Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption
- PMID: 20522596
- PMCID: PMC3279530
- DOI: 10.2337/db09-1293
Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption
Abstract
Objective: It has been proposed that skeletal muscle insulin resistance arises from the accumulation of intramyocellular lipid metabolites that impede insulin signaling, including diacylglycerol and ceramide. We determined the role of de novo ceramide synthesis in mediating muscle insulin resistance.
Research design and methods: Mice were subjected to 12 weeks of diet-induced obesity (DIO), and then treated for 4 weeks with myriocin, an inhibitor of serine palmitoyl transferase-1 (SPT1), the rate-limiting enzyme of de novo ceramide synthesis.
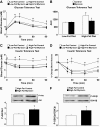
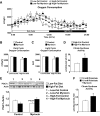
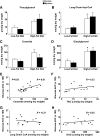
Results: After 12 weeks of DIO, C57BL/6 mice demonstrated a doubling in gastrocnemius ceramide content, which was completely reversed (141.5 ± 15.8 vs. 94.6 ± 10.2 nmol/g dry wt) via treatment with myriocin, whereas hepatic ceramide content was unaffected by DIO. Interestingly, myriocin treatment did not alter the DIO-associated increase in gastrocnemius diacyglycerol content, and the only correlation observed between lipid metabolite accumulation and glucose intolerance occurred with ceramide (R = 0.61). DIO mice treated with myriocin showed a complete reversal of glucose intolerance and insulin resistance which was associated with enhanced insulin-stimulated Akt and glycogen synthase kinase 3β phosphorylation. Furthermore, myriocin treatment also decreased intramyocellular ceramide content and prevented insulin resistance development in db/db mice. Finally, myriocin-treated DIO mice displayed enhanced oxygen consumption rates (3,041 ± 124 vs. 2,407 ± 124 ml/kg/h) versus their control counterparts.
Conclusions: Our results demonstrate that the intramyocellular accumulation of ceramide correlates strongly with the development of insulin resistance, and suggests that inhibition of SPT1 is a potentially promising target for the treatment of insulin resistance.
Figures

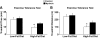
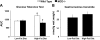

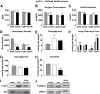
Comment in
-
Targeting ceramide synthesis to reverse insulin resistance.Diabetes. 2010 Oct;59(10):2351-3. doi: 10.2337/db10-0912. Diabetes. 2010. PMID: 20876726 Free PMC article. No abstract available.
References
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 - PubMed
-
- Muoio DM, Newgard CB. Obesity-Related Derangements in Metabolic Regulation. Annu Rev Biochem, 2006 - PubMed
-
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A 2007;104:16480–16485 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

