Differential effects of conjugated linoleic acid isomers on macrophage glycerophospholipid metabolism
- PMID: 20522602
- PMCID: PMC2918450
- DOI: 10.1194/jlr.M007906
Differential effects of conjugated linoleic acid isomers on macrophage glycerophospholipid metabolism
Abstract
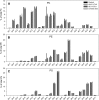
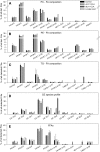
Conjugated linoleic acids (CLA) are dietary fatty acids. Whereas cis-9,trans-11-(c9,t11)-CLA can be found in meat and dairy products, trans-9,trans-11-(t9,t11)-CLA is a constituent of vegetable oils. Previous studies showed that these two isomers activate different nuclear receptors and, thus, expression of genes related to lipid metabolism. Here we show that these CLA isomers are differentially elongated and desaturated in primary monocyte-derived macrophages isolated from healthy volunteers by using gas chromatography-mass spectrometry (GC-MS). We further demonstrate that c9,t11-CLA incorporates in phosphatidylcholine (PC) and phosphatidylethanolamine (PE) species and activates de novo glycerophospholipid synthesis by quantitative electrospray ionization-tandem mass spectrometry (ESI-MS/MS). c9,t11-CLA leads to strong shifts of the species profiles to PC 18:2/18:2 and PE 18:2/18:2, which are due to de novo synthesis and fatty acid remodeling. In contrast, t9,t11-CLA is preferentially bound to neutral lipids, including triglycerides and cholesterol esters. Taken together our results show that c9,t11-CLA and t9,t11-CLA have differential effects on PC and PE metabolism. Moreover, these data demonstrate that the structure of fatty acids not only determines their incorporation into lipid classes but also modulates the kinetics of lipid metabolism, particularly PC synthesis.
Figures


References
-
- Schmitz G., Ecker J. 2008. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 47: 147–155. - PubMed
-
- Kramer J. K., Cruz-Hernandez C., Deng Z., Zhou J., Jahreis G., Dugan M. E. 2004. Analysis of conjugated linoleic acid and trans 18:1 isomers in synthetic and animal products. Am. J. Clin. Nutr. 79: 1137S–1145S. - PubMed
-
- Aldai N., Osoro K., Barron L. J., Najera A. I. 2006. Gas-liquid chromatographic method for analysing complex mixtures of fatty acids including conjugated linoleic acids (cis9trans11 and trans10cis12 isomers) and long-chain (n-3 or n-6) polyunsaturated fatty acids. Application to the intramuscular fat of beef meat. J. Chromatogr. A. 1110: 133–139. - PubMed
-
- Kraft J., Kramer J. K., Schoene F., Chambers J. R., Jahreis G. 2008. Extensive analysis of long-chain polyunsaturated fatty acids, CLA, trans-18:1 isomers, and plasmalogenic lipids in different retail beef types. J. Agric. Food Chem. 56: 4775–4782. - PubMed
-
- Eder K., Ringseis R. 2010. Metabolism and actions of conjugated linoleic acids on atherosclerosis-related events in vascular endothelial cells and smooth muscle cells. Mol. Nutr. Food Res. 54: 17–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

