Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging
- PMID: 20522652
- PMCID: PMC2893674
- DOI: 10.2353/ajpath.2010.090915
Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging
Erratum in
- Am J Pathol. 2010 Oct;177(4):2146. Wuertzer, Charles A [added]
Abstract
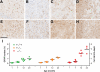
Progranulin (PGRN) is involved in wound repair, inflammation, and tumor formation, but its function in the central nervous system is unknown. Roles in development, sexual differentiation, and long-term neuronal survival have been suggested. Mutations in the GRN gene resulting in partial loss of the encoded PGRN protein cause frontotemporal lobar degeneration with ubiquitin immunoreactive inclusions. We sought to understand the neuropathological consequences of loss of PGRN function throughout the lifespan of GRN-deficient ((-/+) and (-/-)) mice. An aged series of GRN-deficient and wild-type mice were compared by histology, immunohistochemistry, and electron microscopy. Although GRN-deficient mice were viable, GRN(-/-) mice were produced at lower than predicted frequency. Neuropathologically, GRN(-/+) were indistinguishable from controls; however, GRN(-/-) mice developed age-associated, abnormal intraneuronal ubiquitin-positive autofluorescent lipofuscin. Lipofuscin was noted in aged GRN(+/+) mice at levels comparable with those of young GRN(-/-) mice. GRN(-/-) mice developed microgliosis, astrogliosis, and tissue vacuolation, with focal neuronal loss and severe gliosis apparent in the oldest GRN(-/-) mice. Although no overt frontotemporal lobar degeneration with ubiquitin immunoreactive inclusions type- or TAR DNA binding protein-43-positive lesions were observed, robust lipofuscinosis and ubiquitination in GRN(-/-) mice is strikingly similar to changes associated with aging and cellular decline in humans and animal models. Our data suggests that PGRN plays a key role in maintaining neuronal function during aging and supports the notion that PGRN is a trophic factor essential for long-term neuronal survival.
Figures
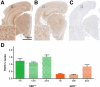
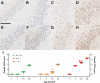

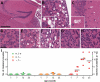
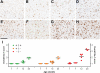

References
-
- Daniel R, Daniels E, He Z, Bateman A. Progranulin (acrogranin/PC cell-derived growth factor/granulin-epithelin precursor) is expressed in the placenta, epidermis, microvasculature, and brain during murine development. Dev Dyn. 2003;227:593–599. - PubMed
-
- Avrova AO, Stewart HE, De Jong WD, Heilbronn J, Lyon GD, Birch PR. A cysteine protease gene is expressed early in resistant potato interactions with Phytophthora infestans. Mol Plant Microbe Interact. 1999;12:1114–1119. - PubMed
-
- He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

