Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination
- PMID: 20522769
- PMCID: PMC3066098
- DOI: 10.1126/science.1188403
Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination
Abstract
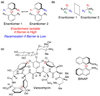
Despite the widespread use of axially chiral, or atropisomeric, biaryl ligands in modern synthesis and the occurrence of numerous natural products exhibiting axial chirality, few catalytic methods have emerged for the direct asymmetric preparation of this compound class. Here, we present a tripeptide-derived small-molecule catalyst for the dynamic kinetic resolution of racemic biaryl substrates. The reaction proceeds via an atropisomer-selective electrophilic aromatic substitution reaction using simple bromination reagents. The result is an enantioselective synthesis that delivers chiral nonracemic biaryl compounds with excellent optical purity and good isolated chemical yields (in most cases a >95:5 enantiomer ratio and isolated yields of 65 to 87%). A mechanistic model is advanced that accounts for the basis of selectivity observed.
Figures


References
-
- Kenner J, Stubbings WVA. J. Chem. Soc. 1921;119:593.
-
- Adams R, Yuan HC. Chem. Rev. 1933;12:261.
-
- Stoughton RW, Adams R. J. Am. Chem. Soc. 1933;54:4426.
-
- Eliel EL, Wilen SH, editors. Stereochemistry of Organic Compounds. New York: Wiley; 1994. pp. 1142–1148.
-
- Seebach D, Jaeschke G, Gottwald K, Matsuda K, Chaplin DA, Breuning M, Bringmann G. Tetrahedron. 1997;53:7539.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

