Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial
- PMID: 20522788
- PMCID: PMC2970846
- DOI: 10.1164/rccm.200912-1853OC
Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial
Abstract
Rationale: Enteral administration of probiotics may modify the gastrointestinal environment in a manner that preferentially favors the growth of minimally virulent species. It is unknown whether probiotic modification of the upper aerodigestive flora can reduce nosocomial infections.
Objectives: To determine whether oropharyngeal and gastric administration of Lactobacillus rhamnosus GG can reduce the incidence of ventilator-associated pneumonia (VAP).
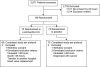
Methods: We performed a prospective, randomized, double-blind, placebo-controlled trial of 146 mechanically ventilated patients at high risk of developing VAP. Patients were randomly assigned to receive enteral probiotics (n = 68) or an inert inulin-based placebo (n = 70) twice a day in addition to routine care.
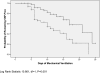
Measurements and main results: Patients treated with Lactobacillus were significantly less likely to develop microbiologically confirmed VAP compared with patients treated with placebo (40.0 vs. 19.1%; P = 0.007). Although patients treated with probiotics had significantly less Clostridium difficile-associated diarrhea than patients treated with placebo (18.6 vs. 5.8%; P = 0.02), the duration of diarrhea per episode was not different between groups (13.2 ± 7.4 vs. 9.8 ± 4.9 d; P = 0.39). Patients treated with probiotics had fewer days of antibiotics prescribed for VAP (8.6 ± 10.3 vs. 5.6 ± 7.8 d; P = 0.05) and for C. difficile-associated diarrhea (2.1 ± 4.8 SD d vs. 0.5 ± 2.3 d; P = 0.02). No adverse events related to probiotic administration were identified.
Conclusions: These pilot data suggest that L. rhamnosus GG is safe and efficacious in preventing VAP in a select, high-risk ICU population. Clinical trial registered with www.clinicaltrials.gov (NCT00613795).
Figures
Comment in
-
Prevention of ventilator-associated pneumonia: bugs or drugs?Am J Respir Crit Care Med. 2010 Oct 15;182(8):993-4. doi: 10.1164/rccm.201007-1033ED. Am J Respir Crit Care Med. 2010. PMID: 20947907 No abstract available.
References
-
- Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH; VAP Outcomes Scientific Advisory Group. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002;122:2115–2121. - PubMed
-
- Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, Jaeschke RZ, Brun-Buisson C. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med 1998;129:433–440. - PubMed
-
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. - PubMed
-
- Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest 2001;120:555–561. - PubMed
-
- Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care 2005;506:714–721. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical