Cardiomyogenesis in the adult human heart
- PMID: 20522802
- PMCID: PMC2987602
- DOI: 10.1161/CIRCRESAHA.110.223024
Cardiomyogenesis in the adult human heart
Retraction in
-
Retraction of: Cardiomyogenesis in the Adult Human Heart.Circ Res. 2019 Feb 15;124(4):e22. doi: 10.1161/RES.0000000000000246. Circ Res. 2019. PMID: 30582463 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern.Circ Res. 2019 Jan 18;124(2):e4-e5. doi: 10.1161/RES.0000000000000241. Circ Res. 2019. PMID: 30582460 No abstract available.
-
Expression of Concern.Circulation. 2019 Jan 15;139(3):e5-e6. doi: 10.1161/CIR.0000000000000639. Circulation. 2019. PMID: 30615475 No abstract available.
Abstract
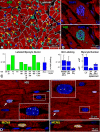
Rationale: The ability of the human heart to regenerate large quantities of myocytes remains controversial, and the extent of myocyte renewal claimed by different laboratories varies from none to nearly 20% per year.
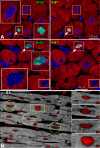
Objective: To address this issue, we examined the percentage of myocytes, endothelial cells, and fibroblasts labeled by iododeoxyuridine in postmortem samples obtained from cancer patients who received the thymidine analog for therapeutic purposes. Additionally, the potential contribution of DNA repair, polyploidy, and cell fusion to the measurement of myocyte regeneration was determined.
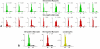
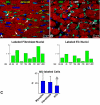
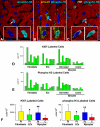
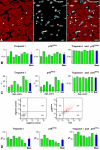
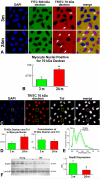
Methods and results: The fraction of myocytes labeled by iododeoxyuridine ranged from 2.5% to 46%, and similar values were found in fibroblasts and endothelial cells. An average 22%, 20%, and 13% new myocytes, fibroblasts, and endothelial cells were generated per year, suggesting that the lifespan of these cells was approximately 4.5, 5, and 8 years, respectively. The newly formed cardiac cells showed a fully differentiated adult phenotype and did not express the senescence-associated protein p16(INK4a). Moreover, measurements by confocal microscopy and flow cytometry documented that the human heart is composed predominantly of myocytes with 2n diploid DNA content and that tetraploid and octaploid nuclei constitute only a small fraction of the parenchymal cell pool. Importantly, DNA repair, ploidy formation, and cell fusion were not implicated in the assessment of myocyte regeneration.
Conclusions: Our findings indicate that the human heart has a significant growth reserve and replaces its myocyte and nonmyocyte compartment several times during the course of life.
Figures



References
-
- Losordo D, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. - PubMed
-
- Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. - PubMed
-
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham HR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. - PubMed
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Cascapera S, Beltrami AP, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. - PMC - PubMed
-
- Hosoda T, D’Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA. 2009;106:17169–17174. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

