Recognition tunneling
- PMID: 20522930
- PMCID: PMC2891988
- DOI: 10.1088/0957-4484/21/26/262001
Recognition tunneling
Abstract
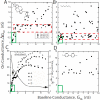
Single molecules in a tunnel junction can now be interrogated reliably using chemically functionalized electrodes. Monitoring stochastic bonding fluctuations between a ligand bound to one electrode and its target bound to a second electrode ('tethered molecule-pair' configuration) gives insight into the nature of the intermolecular bonding at a single molecule-pair level, and defines the requirements for reproducible tunneling data. Simulations show that there is an instability in the tunnel gap at large currents, and this results in a multiplicity of contacts with a corresponding spread in the measured currents. At small currents (i.e. large gaps) the gap is stable, and functionalizing a pair of electrodes with recognition reagents (the 'free-analyte' configuration) can generate a distinct tunneling signal when an analyte molecule is trapped in the gap. This opens up a new interface between chemistry and electronics with immediate implications for rapid sequencing of single DNA molecules.
Figures






Similar articles
-
Physical model for recognition tunneling.Nanotechnology. 2015 Feb 27;26(8):084001. doi: 10.1088/0957-4484/26/8/084001. Epub 2015 Feb 3. Nanotechnology. 2015. PMID: 25650375 Free PMC article.
-
Fixed-gap tunnel junction for reading DNA nucleotides.ACS Nano. 2014 Dec 23;8(12):11994-2003. doi: 10.1021/nn505356g. Epub 2014 Nov 12. ACS Nano. 2014. PMID: 25380505 Free PMC article.
-
Transverse tunneling through DNA hydrogen bonded to an electrode.Nano Lett. 2008 Aug;8(8):2530-4. doi: 10.1021/nl801646y. Epub 2008 Jul 29. Nano Lett. 2008. PMID: 18662039 Free PMC article.
-
Recognition of chemical identity of organic adsorbates on solid surfaces at the nanoscale by molecular STM tips.Anal Sci. 2010;26(10):1023-32. doi: 10.2116/analsci.26.1023. Anal Sci. 2010. PMID: 20953043 Review.
-
Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy.Chem Soc Rev. 2003 May;32(3):139-50. doi: 10.1039/b206566p. Chem Soc Rev. 2003. PMID: 12792937 Review.
Cited by
-
A Review on Perception of Binding Kinetics in Affinity Biosensors: Challenges and Opportunities.ACS Omega. 2025 Jan 27;10(5):4197-4216. doi: 10.1021/acsomega.4c10040. eCollection 2025 Feb 11. ACS Omega. 2025. PMID: 39959045 Free PMC article. Review.
-
Identifying single bases in a DNA oligomer with electron tunnelling.Nat Nanotechnol. 2010 Dec;5(12):868-73. doi: 10.1038/nnano.2010.213. Epub 2010 Nov 14. Nat Nanotechnol. 2010. PMID: 21076404 Free PMC article.
-
Gap distance and interactions in a molecular tunnel junction.J Am Chem Soc. 2011 Sep 14;133(36):14267-9. doi: 10.1021/ja2067737. Epub 2011 Aug 18. J Am Chem Soc. 2011. PMID: 21838292 Free PMC article.
-
Classification of DNA nucleotides with transverse tunneling currents.Nanotechnology. 2017 Jan 6;28(1):015502. doi: 10.1088/0957-4484/28/1/015502. Epub 2016 Nov 29. Nanotechnology. 2017. PMID: 27897144 Free PMC article.
-
Can One Define the Conductance of Amino Acids?Biomolecules. 2019 Oct 7;9(10):580. doi: 10.3390/biom9100580. Biomolecules. 2019. PMID: 31591358 Free PMC article.
References
-
- Hamers RJ, Tromp RM, Demuth JE. Surface Electronic Structure of Si (111)-(7×7) Resolved in Real Space. Phys. Rev. Lett. 1986;56:1972–1975. - PubMed
-
- Hipps KW. Handbook of Applied Solid State Spectroscopy. Kluwer; Berlin: 2005. Scanning Tunneling Spectroscopy. in press.
-
- Shapir E, Cohen H, Calzolari A, Cavazzoni C, Ryndyk DA, Cuniberti G, Kotlyar A, Felice RD, Porath D. Electronic structure of single DNA molecules resolved by transverse scanning tunnelling spectroscopy. Nature Materials. 2008;7:68–74. - PubMed
-
- Tanaka H, Kawai T. Partial sequencing of a single DNA molecule with a scanning tunnelling microscope. Nature Nanotechnology. 2009;4:518–522. - PubMed
-
- Kuznetsov AM, Ulstrup J. Electron transfer in chemistry and biology. Wiley; New York: 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
